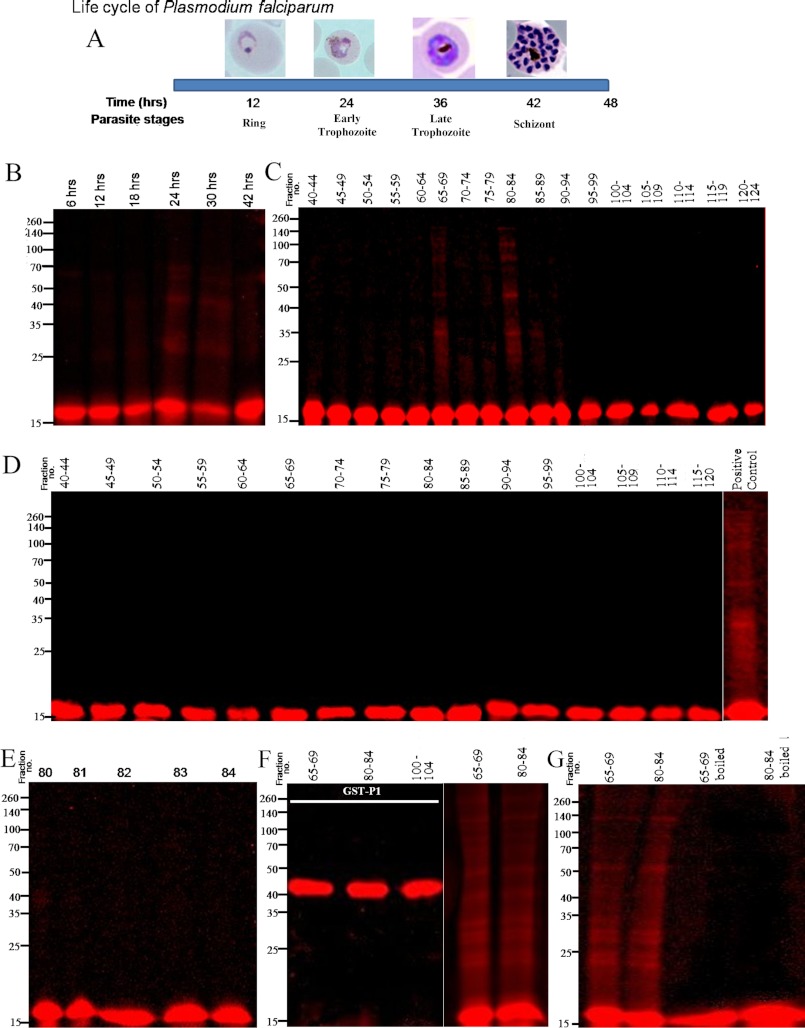
FIGURE 6.
Probing in vitro oligomerization of tetramethylrhodamine-labeled rPfP2 protein using parasite extracts. A, schematic depiction of the time scale of P. falciparum parasite development in erythrocyte. In a 48-h life cycle, the parasite changes its morphology from ring, trophozoite to multinucleated schizont stages. All subsequent panels depict reactions where 1 ng of tetramethylrhodamine-conjugated rPfP2 protein was incubated in 40 μl of various parasite protein preparations at 37 °C for 3 h. The mixture was treated with SDS loading dye and resolved on a 12% SDS-PAGE under reducing conditions. B, labeled rPfP2 was incubated with 4 μg of parasite protein extracts from each of 6-, 12-, 18-, 24-, 30-, and 42-h synchronized P. falciparum cultures. C, labeled rPfP2 was incubated with 25 μl of pooled gel filtration fractions obtained from 30-h parasite cultures Fig. 4C. D, labeled rPfP2 was incubated with 25 μl of pooled gel filtration fractions obtained from 18-h parasite cultures (Fig. 4A). E, labeled rPfP2 was incubated with 5 μl of individual gel filtration fractions (number 80–84) from 30-h parasite culture Fig. 4C. F, rhodamine-labeled recombinant GST-PfP1 (24) protein was incubated with 25 μl of pooled gel filtration fractions (65–69 and 80–84) obtained from 30-h PMI parasite cultures Fig. 4C. Labeled rPfP2 was used a positive control. G, about 25 μl of the two pooled gel filtration fractions (65–69 and 80–84) were boiled for 10 min. The supernatant was incubated with rhodamine-labeled rPfP2. Rhodamine-labeled rPfP2 incubated with the respective fractions without boiling was used as a control.