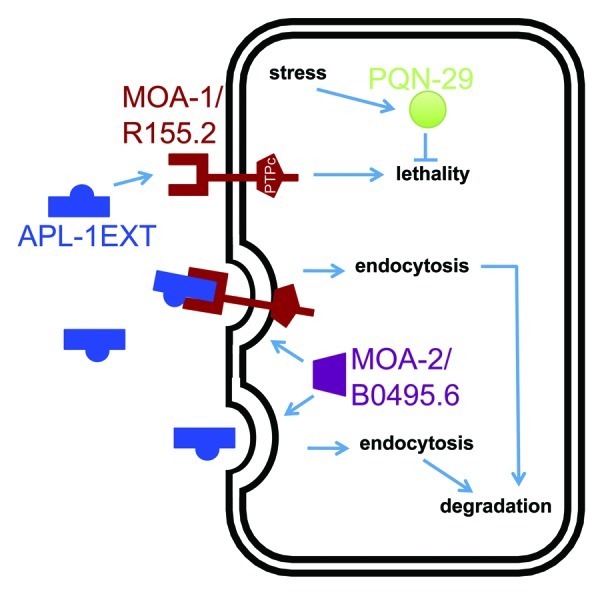
Figure 2. One model for the pathway underlying the temperature-sensitive apl-1(yn5) lethality. apl-1(yn5) mutants only produce the extracellular domain of APL-1 (APL-1EXT), which is presumably released into the extracellular space to act on distant cells. The temperature-sensitive embryonic lethality of apl-1(yn5) mutants is suppressed by the moa-1(yn38) mutation and is enhanced by the moa-2(yn39) mutation and by RNAi against pqn-29.1 Based on their predicted protein domains and phenotypes, we suggest that APL-1EXT (and presumably sAPL-1) binds to MOA-1/R155.2 RPTP. MOA-2/B0495.6 could facilitate endocytosis of APL-1EXT bound to its receptor for degradation by lysosomes. An increase in temperature induces a stress response in C. elegans and may upregulate PQN-29 to attenuate the apl-1(yn5)-induced lethality.
