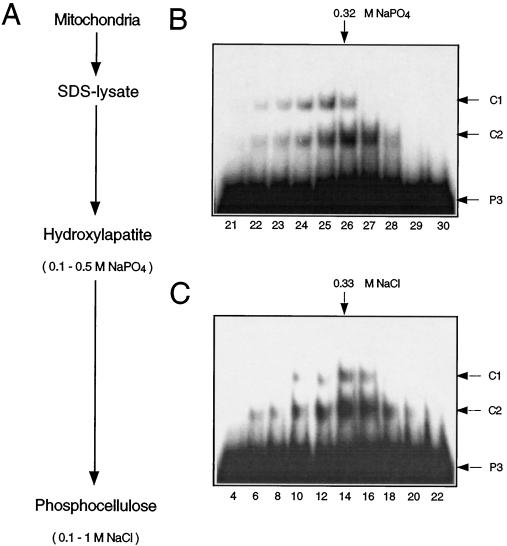
Figure 1.
Purification of DNA-binding proteins from pea mitochondria. A, Purification scheme. Pea mitochondria were denatured by boiling in an SDS-containing buffer. The SDS-lysate was loaded onto a hydroxyapatite column and eluted with a linear NaPO4 gradient (0.1–0.5 m). Aliquots of the eluted fractions were renatured and tested for DNA-binding activity in mobility-shift experiments (B). Fractions with binding activity (nos. 22–28) were pooled, renatured, and loaded onto a phosphocellulose column. The column was eluted with a linear NaCl gradient (0.1–1 m), and fractions were assayed for DNA-binding activity by mobility-shift analysis (C). The approximate NaPO4 and NaCl concentrations of fractions with the highest binding activities are indicated at the upper margins. Arrows in the right margins indicate the positions of the unbound ds-oligonucleotide P3 and of the DNA-protein complexes C1 and C2. Fraction numbers are given at the bottom in B and C.