Abstract
Background:
In congenital adrenal hyperplasia (CAH), long-term glucocorticoid treatment coupled with increased androgens may lead to undesirable metabolic effects. The aim of our report was to determine the prevalence of metabolic abnormalities and cardiovascular risk factors in a population of adult patients with CAH due to 21 hydroxylase deficiency.
Materials and Methods:
Twenty-six patients (11 males and 15 females, mean age ± SD=27.4±8.2 years) were recruited. Anthropometry, body composition, metabolic parameters and cardiovascular risk factors were studied.
Results:
Obesity (overweight included) was noted in 16 patients (61.5%), with android distribution in all cases. Bioelectrical impedance showed increased body fat mass in 12 patients (46.1%). Lipid profile alterations and carbohydrate metabolism disorders were detected in seven (26.9%) and five (19.2%) patients respectively. Moderate hepatic cytolysis, associated with hepatic steatosis, was found in one patient. Seven patients (27%) had insulin resistance. Ambulatory blood pressure monitoring showed abnormalities in six patients (23%). Increased carotid intima media thickness was found in 14 patients (53.8%).
Conclusion:
Adult CAH patients tend to have altered metabolic parameters and a higher prevalence of cardiovascular risk factors. Lifelong follow-up, lifestyle modifications, and attempts to adjust and reduce the glucocorticoid doses seem important.
Keywords: Cardiovascular risk, metabolic profile, body composition, congenital adrenal hyperplasia, 21-hydroxylase deficiency
INTRODUCTION
Congenital adrenal hyperplasia (CAH) describes a group of inherited autosomal recessive disorders affecting adrenal steroid synthesis. The most frequent CAH variant, accounting for 95% of all affected patients, is 21-hydroxylase deficiency (21-OHD) and caused by inactivating mutations in the 21-hydroxylase gene (CYP21A2).[1] The impaired cortisol secretion causes ACTH levels to rise and stimulate adrenocortical hormone secretion, resulting in adrenal hyperplasia, and increased production of androgens and steroid precursors before the enzymatic defect. Two distinct phenotypes are recognized in CAH due to 21-OHD: classical CAH, the most severe form comprises both salt-wasting (SW) and simple virilizing (SV) forms, with a worldwide incidence of 1:15000 live births, and the nonclassical (NC) form which may be asymptomatic or associated with signs of postnatal or even adult onset androgen excess. Most patients are compound heterozygotes having different mutations of the CYP21 gene on each allele.[1] The clinical expression of CAH is reported to be correlated with the less severely mutated allele.[2] Treatment of CAH consists of glucocorticoids and, when necessary, mineralocorticoids to prevent adrenal crises and to suppress the abnormal secretion of androgens and steroid precursors from the adrenal cortex.[3] In addition to impaired adrenocortical function, classic CAH is characterized by compromised adrenomedullary function. The latter is owing to developmental defects in the formation of adrenal medulla, leading to a reduction in epinephrine and metanephrine stores.[4]
Lifelong glucocorticoid replacement therapy is required in CAH patients to reduce adrenal androgen excess.[5] The therapeutic spectrum of glucocorticoids is narrow and supraphysiological doses, often needed to control the hyperandrogenism, are potentially harmful and result in the development of an iatrogenic Cushing's syndrome.[6] Abnormalities in lipid and glucose metabolism, as well as increased body mass index (BMI), body fat mass, insulin, leptin and blood pressure levels have been reported in patients with 21-OHD, with potential long-term complications in later life.[7,8]
The aim of the present work was to determine the prevalence of metabolic abnormalities and cardiovascular risk factors in a population of adult patients with CAH due to 21-OHD.
MATERIALS AND METHODS
Patients
We included 26 patients (11 males and 15 females, mean age±SD=27.4±8.2 years, range=16.5-48 years) who were followed at the department of endocrinology in Mahdia and Sfax, in Tunisia. This sample includes all patients with CAH due to 21-OHD, aged more than 16 years and regularly followed at these two clinics since 1982.
Patients or their parents were informed about the purpose of the study. Clinical and hormonal data of recruited patients are presented in Table 1. Data concerning presentation, diagnosis, medical and surgical treatment, and other relevant information were reviewed. All individuals had CAH with 21-OHD. The diagnosis of CAH was based on clinical and biochemical criteria (i.e. elevated levels of 17-hydroxyprogesterone (17- OHP) and androstenedione, ACTH stimulation test). None of the patients was treated in utero with dexamethasone.
Table 1.
Clinical and hormonal data of the included patients
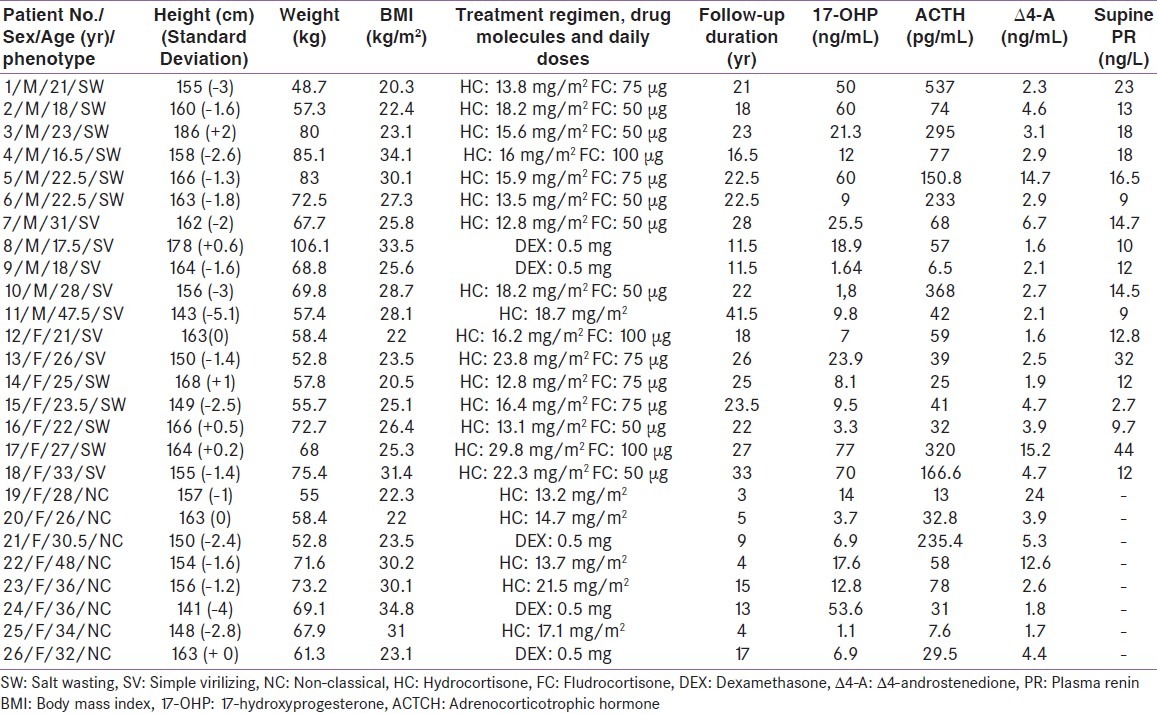
Ten patients (six males, four females) had the SW form of CAH, and they had been diagnosed in their first year of life. Eight patients (five males, three females, age at diagnosis: Between birth and six years) were diagnosed as classical SV patients. In the other eight patients (women only in this group), the NC form was diagnosed during between 15 and 44 years of age. All patients had been treated from the time of diagnosis. Twenty-one patients, 16 with the classical form and five with the NC form, were started on a regimen of hydrocortisone (HC), given two or three times daily, while the remaining five patients (three with the NC form and two with the classic SV form) were treated with dexamethasone (once daily). Salt wasters were treated with 27.9±9.6mg/m2 per day of HC the first two years of life, and the doses were decreased to 17.6±6.6mg/m2 per day during childhood. Daily doses of HC in patients with classical and NC form were respectively 17.3±4.6mg/m2 and 16.04±3.4mg/m2 during adulthood. For dexamethasone, the prescribed doses ranged from 0.25 to 0.75mg per day. Fifteen patients (10 with the SW form and five with the SV form) additionally received 9α-fludrocortisone (FC; twice daily).
The adequacy of therapy was monitored periodically on the basis of clinical and laboratory data, in accordance with current guidelines.[9] Adequate hormonal control of CAH was defined if serum androgen levels were within the normal range and 17-OHP concentration in serum was 2.0-10ng/mL (6-30nmol/L). The mean duration of the follow-up period was 18.5±9.3 (range 3 to 41.5) years. Good compliance with glucocorticoid treatment was noted in 14 patients (53.8%). Seven patients (five with the SW form and two with the SV form) had experienced a salt-wasting adrenal crisis in the neonatal period. The onset of puberty (defined by onset of breast development in females and increased testicular Volumes in males) was 10.8±1.4 years for girl and 11.2±1.2 years for boys. Menarche occurred spontaneously in all patients, at an average age of 12.8±1.1 years.
One female patient with the NC form (patient n°23) had a history of previously diagnosed congenital hypothyroidism. She was started on L-thyroxine 10μg/kg per day at the age of six months, but she was irregular in follow-up and was not compliant with treatment.
One patient with the SW form (patient n°4) developed hypertension at eight year of age and responded well to Nifedipine. Detailed investigations (renal Doppler ultrasound, adrenal CT scan, urinary metanephrine, 11-deoxycortisol and aldosterone concentrations) failed to detect any cause for secondary hypertension, and a diagnosis of essential hypertension was made.
Methods
From January 2009 to November 2010, patients were prospectively studied as outpatients.
Clinical characteristics and body composition
Height, weight, and waist circumference were evaluated using standard methods. Blood pressure, supine and standing, was registered and signs of hypo/hypercortisolism and hyperandrogenism were recorded. Body mass index (BMI) was calculated as weight/(height)2 (kg/m2). Fat mass (kg) and body fat percent (BF%) were assessed with bioelectrical impedance equipment (TANITA, TBF-300, France). The measurements were performed in a standing position, with electrodes in contact with soles and heels of both feet. The equation for BF% used by this model of Tanita was developed by Brožek: BF%=(4.57/body density - 4.142) * 100.[10]
Endocrinological and metabolic investigations
Blood samples were collected in the morning after an overnight fast for measurements of serum lipids [total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C)], liver enzymes [serum alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transpeptidase (GGT)], electrolytes, plasma glucose, hormones [insulin, leptin, testosterone, Δ4-androstenedione (Δ4-A), 17-OHP, renin, plasma ACTH]. Samples were centrifuged and separated immediately after collection and were stored at −20°C until assayed.
Biochemical assays
Plasma glucose was measured using a glucose oxidase method on a glucose analyzer and TC, TG, and HDL-C by enzymatic methods. Fasting serum insulin levels were measured by enzyme linked immunosorbent assay (ELISA) using the insulin ELISA kit (biocompare, Catalog Number YK060; intraassay and interassay coefficients of variation <10%). Serum leptin levels were determined by ELISA technique using Leptin-EASIA KAP2281 kit (DIA source ImmunoAssays S.A. B-1400 Nivelles, Belgium), with a sensitivity of 0.5 ng/mL.
Metabolic assessment
A 75g oral glucose tolerance test (OGTT) was done in all patients and was evaluated using the criteria of the World Health Organization (WHO).[11]
Low-density lipoprotein cholesterol (LDL-C) was calculated using Friedwald's formula in individuals with triglycerides <400mg/dL. The following situations were defined as dyslipidemia: TC >200mg/dL, LDL-C > 135mg/dL, TG >150mg/dL, and/or HDL-C <35mg/dL for males or <40mg/dL for females.[12]
Insulin resistance (IR) was estimated using the homeostasis model assessment (HOMA) method as previously described: IR=insulin (μU/mL) x glucose (mmol/L)/22.5.[13] A HOMA-index ≥2.77 has been suggested to indicate insulin resistance.[14] The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATPIII) criteria were used to define the metabolic syndrome.[15]
Cardiovascular risk profile assessment
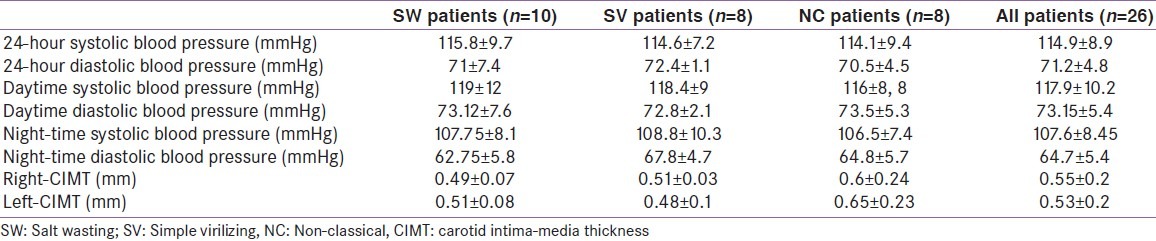
Ambulatory 24hrs blood pressure profiles (ABPM) was performed in all patients.The following blood pressure (BP) characteristics were measured: Mean 24hrs systolic and diastolic, daytime systolic and diastolic, and night-time systolic and diastolic. Daytime BP less than or equal to 140/90mmHg and the night time blood pressure less than or equal to 125/80mmHg were considered to be normal.[16,17] Patients were defined as dippers when nighttime systolic BP fall was ≥10%, and non-dippers when nighttime systolic BP fall was <10%.[18]
Carotid Intima-Media Thickness (CIMT) was assessed in all patients using high-resolution B-mode ultrasound with a 10-MHz linear transducer. Measurements were performed in the posterior wall of both common carotid arteries (CCA), at three angles. The average of the mean CIMT values of the three segments was calculated to determine the mean right and left CIMT per patient.[19] In the current study, the mean CIMT was compared with reference values from the Multi-Ethnic Study of Atherosclerosis.[20] Patients were thereby stratified as having normal CIMT (CIMT <75th percentile) or increased CIMT (CIMT ≥75th percentile in at least one CCA).[20]
RESULTS
Clinical characteristics and body composition
Mean female height was 158.8±7.6 cm (range, 141-168) and mean male height was 163.1±11.4 cm (range, 143- 186). Twenty-one patients (nine males and 12 females, 80.7%) had a final height below the target height. Mean BMI was 26.5±4.3 kg/m2 (range, 20.3-34.8). Mean office systolic and diastolic blood pressures were 124±17 and 87±11mmHg respectively (range, 100-150 and 40-100 respectively), and there was no significant change in values on standing up. Except of patient n°4 with prior diagnosis of hypertension, no one has been diagnosed with office hypertension. No patient had signs of hypercortisolism. Bioelectrical impedance showed increased body fat mass in 12 patients (46.1%) including nine (SW/SV: 5/4) with the classical form and three with the NC form.
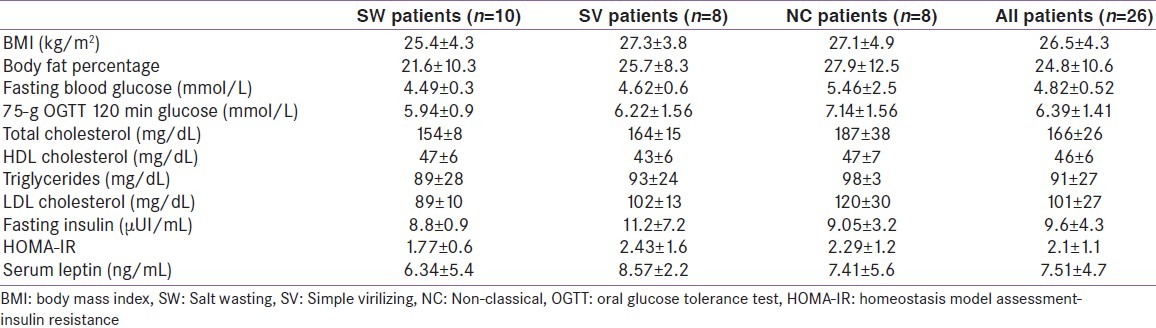
Metabolic profile
10 patients (three males and seven females, 38.4%) had normal BMI; eight patients (five males and three females, 30.7%) were overweight, while eight patients (three males and five females, 30.7%) were obese, with android distribution in all cases. Among all overweight and obese patients, 12 had the classic form (SW/SV: 6/6) and four had the NC form.
Lipid profile alterations (at least one abnormal lipid fraction) were detected in seven patients (one male and six females, 26.9%) including one with the SV form (patient n°8) and six with the NC form. Reduced HDL-C constituted the highest abnormality (five patients; one male and four females) followed by hypertriglyceridemia in three patients (patient n°19, 23 and 24), hypercholesterolemia in two patients (patient n°22 and 25) and elevated LDL-C in 1 patient (patient n°25).
Insulin resistance (HOMA-IR ranging from 2.79 to 4.27) was documented in seven patients (three males and four females, 27%) including one with the SW form (patient n°4), three with the SV form (patient n°8, 9 and 18) and three with the NC form (patient n°22, 23 and 25). Hyperleptinemia was detected in three patients (patient n°2, 15 and 19, 11.5%). Moderate hepatic cytolysis was found in patient n°8 (ASAT: 103 UI/L, ALAT: 223 UI/L). This patient had also hepatic steatosis found on abdominal CT scan that showed abnormal low signal intensity of the liver, hypointense relative to the spleen [Figure 1].
Figure 1.
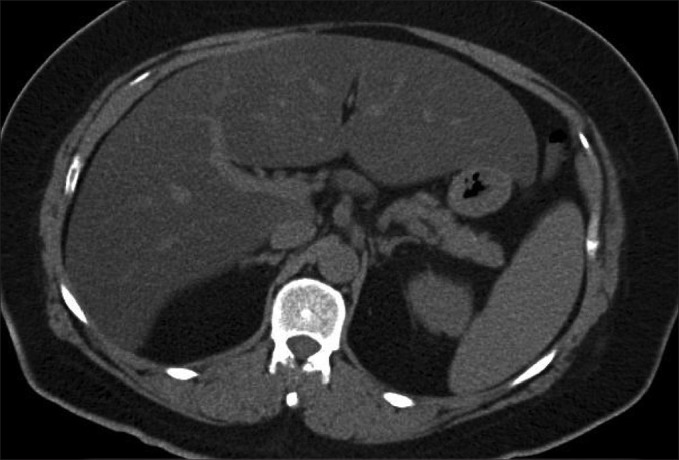
Abdominal CT scan image of patient n°8, with the classical simple virilizing form of congenital adrenal hyperplasia. This image illustrates low density of the hepatic parenchyma (relative tosplenic parenchyma) indicating diffuse steatosis
During OGTT, 21 patients (80.7%) had normal glucose tolerance, while carbohydrate metabolism disorders were detected in five patients (19.2%). Four patients (patients n°8, 22, 24 and 25) were identified as having glucose intolerance and one patient (patient n°22) had diabetes (Blood glucose 120 min: 11.05 mmol/L). The metabolic syndrome as defined by the NCEP ATP III criteria was documented in patient n°23, who had diabetes, obesity, low HDL-C, and hypertriglyceridemia. Table 2 shows metabolic parameters according to clinical phenotype of CAH.
Table 2.
Metabolic profile of patients according to clinical phenotype of congenital adrenal hyperplasia
Cardiovascular risk profile
ABPM showed mild daytime systolic hypertension (150 mmHg) in one patient with the SW form (patient n°5). Indeed, five patients (two males and three females, 19.23%) were classified as nondippers [Figure 2]. Among these patients, one had the SW form, two had the SV form and two had the NC form.
Figure 2.
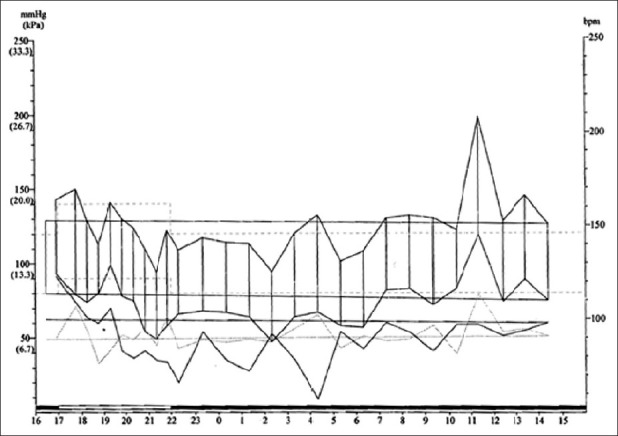
Ambulatory blood pressure monitoring illustrating a nondipper pattern in patient n°8 with the simple virilizing form of congenital adrenal hyperplasia
Comparison with reference values revealed normal CIMT in 12 patients (46.1%). In the remaining 14 patients (five males and nine females, 53.8%), an increased CIMT value, up to 1.1 mm, was observed in at least one CCA. These patients were classified as follow: four with the SW form, four with the SV form and six with the NC form. Table 3 illustrates cardiovascular parameters according to clinical phenotype of CAH.
Table 3.
Prevalence of cardiovascular risk factors in our patients according to clinical phenotype of congenital adrenal hyperplasia
DISCUSSION
We studied cardiovascular and metabolic risk profiles in a cohort of CAH adult patients due to 21-OHD. Our report illustrates some indications of increased cardiovascular and metabolic risk in this population, consistent with the previous studies.[7,8,21]
In our study, seven patients (27%) were overweight, and eight patients (30.7%) were obese, with android distribution in all cases. BMI was found to be elevated in most[5,22,23] but not all reports[24] on CAH patients. Moreover, higher body fat mass has been reported, using different methods such as skin-fold thickness, whole-body dual-energy x-ray absorption (DXA) scan, and bioelectrical impedance analysis.[21,25,26] This last method was used in our study and showed elevated body fat mass in 46.1% of patients. The causes of the excessive increase in body weight and fat mass are unclear, but several factors may contribute. Overtreatment with glucocorticoids may lead to a Cushingoid syndrome with central obesity, resulting from redistribution of fat from peripheral to central depots.[7,8,26] Furthermore, adrenomedullary dysfunction with decreased secretion of epinephrine may play a role in the development of obesity. Catecholamines, like epinephrine, contribute to lipolysis and inhibit insulin secretion, thereby preventing an increase in fat mass. Other possible explanations for the increased fat mass could be hypogonadism in men and hyperandrogenism in women.[7,8,21]
Literature data suggest that both classic and non-classic CAH patients exhibit higher fasting serum insulin and a higher insulin resistance, as determined by HOMA model.[27–29] This appeared to be in line with our findings, since we have found elevated HOMA-IR in 27% of our patients. In addition, elevated serum leptin levels were reported in CAH patients.[29] Recently, Völkl et al found decreased soluble leptin receptor (sOB-R) associated with normal serum leptin concentrations. They hypothesized that this specific change in the leptin axis may contribute to the increased risk of overweight and obesity.[30] In our cohort, only three patients showed hyperleptinemia. So, this finding highlights the importance of measurement of sOB-R in our population.
It has been suggested that high insulin combined with increase leptin concentrations in CAH patients might be due to both overproduction of androgens and treatment with supraphysiological doses of glucocorticoids. The chronic adrenomedullary hypofunction might also promote such metabolic abnormalities as a result of the absence of inhibition receptor β3-mediated catecholamine effects on insulin and leptin secretion.[7,8,21]
Both hyperleptinemia and hyperinsulinism may alter the activity of enzymes participating in adrenal steroidogenesis and may result in a further increase in androgen production and onset of metabolic syndrome.[21] The later was present in only one patient in our study.
Carbohydrate metabolism disorders were detected in five of our participants (19.2%), including four patients with glucose intolerance and one patient with diabetes. In literature, no study has demonstrated an increased frequency of type two diabetes in CAH patients. However, female patients seem to be at increased risk of gestational diabetes mellitus which is a well-known predictor of future type 2 diabetes.[22,31]
To date, only six studies report on lipid profiles in CAH patients.[7,32] Most of them did not show unfavorable changes. Our results, showing lipid profile alterations in 26.9% of patients, disagree thus with literature findings. Normality of lipide profiles in most studies seems to be surprising, because obesity and insulin resistance, both commun in CAH patients, are known to be associated with dyslipidemia. A possible explanation is that lipid profiles were mainly evaluated in younger CAH patients.[7] Nevertheless, the recent study, reported by Zimmermann et al, suscites particular interest. In fact, it was able de demonstrate that, despite apparently normal routinely assessed lipid parameters, CAH patients could have discrete alterations in lipid metabolism characterized by an elevation in concentrations of cholesterol in the small-dense low-density lipoproteins subfractions (sd-LDL). Such alterations might be an additional metabolic risk predisposing to an accelerated atherogenesis.[32]
One of our patients showed moderate hepatic cytolysis, associated with hepatic steatosis. Falhammer et al, demonstrated that obese and non-obese female patients with CAH showed higher liver function tests (LFT) compared with controls. LFT were positively correlated to BMI, body fat and waist circumference. The authors concluded that not only central obesity but also glucocorticoids might influence LFT. Elevated liver enzymes could represent markers for increased future metabolic risk and could further predict the metabolic risk of CAH individuals. However, the prevalence of hepatic steatosis was not determined in this study.[33]
There is minimal data on hypertension in CAH patients.[7,8] Overall, there is tendancy towards an increased prevalence of hypertension. In a previous study including 91 children and adolescents with classic CAH, essential hypertension (hypertension of unknown etiology) was noted in five of them, possibly due to intrinsic dysregulation inherent to CAH.[34] In our study, one patient with the SW form was diagnosed as having essential hypertension after careful investigations. Moreover, altered 24-h blood pressure profiles with elevated systolic levels have been reported. These blood pressure alterations, found in 23% of our patients, seem to be associated with BMI, and not affected by mineralocorticoid or glucocorticoid replacement dose.[35–37] Interestingly, non-dipper blood pressure pattern, characterized by the absence of physiologic nocturnal nadir in systolic blood pressure, has been described in CAH patients.[34–37] Its prevalence was 19, 23% in our patients. Nondipper status has been shown to be an additional risk factor for the development of adverse cardiovascular events.[38]
Our study provides evidence showing a high rate of Increased CIMT in CAH patients. This finding is in line with an Italian study,[39] which demonstrates reduced insulin sensitivity and increased intima-media thickness (IMT), independently of cumulative glucocorticoid doses and androgen levels.[40] IMT is considered as a measure of subclinical atherosclerosis and a predictor of myocardial infarction and stroke, independently of traditional risk factors for atherosclerosis.[40]
Inflammation plays an important role in the initiation and progression of atherosclerosis and the development of atherosclerotic events.[41] Inflammatory markers, such as adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein, have been shown to predict future cardiovascular events in individuals with and without established cardiovascular disease.[41] These markers have not yet been studied in CAH patients.[7,8]
The present study has potential limitations. The small sample and the involvement of few individuals >30 years old did not allow us to establish statistical correlations. In addition, our study cohort appears to be heterogenous since we have included patients with both classic and non-classic forms of the disease. As consequence, it is not surprising to find more higher degrees of obesity, abnormal lipids, and carotid intima-media thickness in the non-classic CAH patients, as this group have received the most potent steroid dexamethasone in the doses almost equal to or higher than the doses used for the classical CAH patients. Despite these limitations, our study was able to assess simultaneously several cardiometabolic risk factors in CAH patients. Further studies with larger cohorts of patients are required in order to better evaluate the risk of atherosclerotic vascular disease in CAH patients, and to discuss the range of pharmacological interventions in this population.
CONCLUSION
The present study illustrates high prevalence of some metabolic abnormalities and established risk factors for cardiovascular disease in CAH adult patients. This unfavorable cardiometabolic profile may be due to either the existence of hyperandrogenism in untreated or undertreated patients or to the supra-physiological doses of glucocorticoids used to suppress androgen levels to normal values. Lifelong followup, early lifestyle modifications, and attempts to adjust the glucocorticoid doses may reduce cardiovascular risk in this group of patients.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–91. doi: 10.1210/edrv.21.3.0398. [DOI] [PubMed] [Google Scholar]
- 2.Forest MG, Tardy V, Nicolino M, David M, Morel Y. 21-Hydroxylase deficiency: An exemplary model of the contribution of molecular biology in the understanding and management of the disease. Ann Endocrinol (Paris) 2005;66:225–32. doi: 10.1016/s0003-4266(05)81754-8. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvie CM, Crouch NS, Rumsby G, Creighton SM, Liao LM, Conway GS. Congenital adrenal hyperplasia in adults: A review of medical, surgical and psychological issues. Clin Endocrinol (Oxf) 2006;64:2–11. doi: 10.1111/j.1365-2265.2005.02410.x. [DOI] [PubMed] [Google Scholar]
- 4.Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343:1362–8. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 5.King JA, Wisniewski AB, Bankowski BJ, Carson KA, Zacur HA, Migeon CJ. Long-term corticosteroid replacement and bone mineral density in adult women with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91:865–9. doi: 10.1210/jc.2005-0745. [DOI] [PubMed] [Google Scholar]
- 6.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–21. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mooij CF, Kroese JM, Claahsen-van der Grinten HL, Tack CJ, Hermus AR. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clin Endocrinol (Oxf) 2010;73:137–46. doi: 10.1111/j.1365-2265.2009.03690.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim MS, Merke DP. Cardiovascular disease risk in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Semin Reprod Med. 2009;27:316–21. doi: 10.1055/s-0029-1225259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joint LWPES/ESPE CAH Working Group. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab. 2002;87:4048–53. doi: 10.1210/jc.2002-020611. [DOI] [PubMed] [Google Scholar]
- 10.Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: Revision of some quantitative assumptions. Ann N Y Acad Sci. 1963;110:113–40. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 11.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23:1108–12. doi: 10.2337/diacare.23.8.1108. [DOI] [PubMed] [Google Scholar]
- 12.Cardiovascular disease risk factors: New areas for research. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1994;841:1–53. [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment:Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Radikova Z, Koska J, Huckova M, Ksinantova L, Imrich R, Vigas M, et al. Insulin sensitivity indices: A proposal of cut-off points for simple identification of insulin-resistant subjects. Exp Clin Endocrinol Diabetes. 2006;114:249–56. doi: 10.1055/s-2006-924233. [DOI] [PubMed] [Google Scholar]
- 15.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: Adjustments and options. Am J Cardiol. 2005;96:53E–59E. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, et al. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation. 2007;115:2145–52. doi: 10.1161/CIRCULATIONAHA.106.662254. [DOI] [PubMed] [Google Scholar]
- 17.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 18.Liivak K, Tillmann V. 24-hour blood pressure profiles in children with congenital adrenal hyperplasia on two different hydrocortisone treatment regimens. J Pediatr Endocrinol Metab. 2009;22:511–7. doi: 10.1515/jpem.2009.22.6.511. [DOI] [PubMed] [Google Scholar]
- 19.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force.Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189-90. [DOI] [PubMed] [Google Scholar]
- 20.Denarié N, Gariepy J, Chironi G, Massonneau M, Laskri F, Salomon J, et al. Distribution of ultrasonographically-assessed dimensions of common carotid arteries in healthy adults of both sexes. Atherosclerosis. 2000;148:297–302. doi: 10.1016/s0021-9150(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 21.Charmandari E, Chrousos GP. Metabolic syndrome manifestations in classic congenital adrenal hyperplasia: Do they predispose to atherosclerotic cardiovascular disease and secondary polycystic ovary syndrome? Ann N Y Acad Sci. 2006;1083:37–53. doi: 10.1196/annals.1367.005. [DOI] [PubMed] [Google Scholar]
- 22.Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:110–6. doi: 10.1210/jc.2006-1350. [DOI] [PubMed] [Google Scholar]
- 23.Völkl TM, Simm D, Beier C, Dörr HG. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 2006;117:E98–105. doi: 10.1542/peds.2005-1005. [DOI] [PubMed] [Google Scholar]
- 24.Gussinyé M, Carrascosa A, Potau N, Enrubia M, Vicens-Calvet E, Ibáñez L, et al. Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics. 1997;100:671–4. doi: 10.1542/peds.100.4.671. [DOI] [PubMed] [Google Scholar]
- 25.Isguven P, Arslanoglu I, Mesutoglu N, Yildiz M, Erguven M. Bioelectrical impedance analysis of body fatness in childhood congenital adrenal hyperplasia and its metabolic correlates. Eur J Pediatr. 2008;167:1263–8. doi: 10.1007/s00431-007-0665-y. [DOI] [PubMed] [Google Scholar]
- 26.Falhammar H, Filipsson Nyström H, Wedell A, Thorén M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2011;164:285–93. doi: 10.1530/EJE-10-0877. [DOI] [PubMed] [Google Scholar]
- 27.Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, Samara D, et al. Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. 2007;67:268–76. doi: 10.1159/000098017. [DOI] [PubMed] [Google Scholar]
- 28.Saygili F, Oge A, Yilmaz C. Hyperinsulinemia and insulin insensitivity in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: The relationship between serum leptin levels and chronic hyperinsulinemia. Horm Res. 2005;63:270–4. doi: 10.1159/000086363. [DOI] [PubMed] [Google Scholar]
- 29.Charmandari E, Weise M, Bornstein SR, Eisenhofer G, Keil MF, Chrousos GP, et al. Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: Potential clinical implications. J Clin Endocrinol Metab. 2002;87:2114–20. doi: 10.1210/jcem.87.5.8456. [DOI] [PubMed] [Google Scholar]
- 30.Völkl TM, Simm D, Körner A, Rascher W, Kiess W, Kratzsch J, et al. Does an altered leptin axis play a role in obesity among children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency? Eur J Endocrinol. 2009;160:239–47. doi: 10.1530/EJE-08-0770. [DOI] [PubMed] [Google Scholar]
- 31.Falhammar H, Davis B, Bond D, Sinha AK. Maternal and neonatal outcomes in the Torres Strait Islands with a sixfold increase in type 2 diabetes in pregnancy over six years. Aust N Z J Obstet Gynaecol. 2010;50:120–6. doi: 10.1111/j.1479-828X.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann A, Grigorescu-Sido P, AlKhzouz C, Patberg K, Bucerzan S, Schulze E, et al. Alterations in lipid and carbohydrate metabolism in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2010;74:41–9. doi: 10.1159/000313368. [DOI] [PubMed] [Google Scholar]
- 33.Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjöld A, Hagenfeldt K, et al. Increased liver enzymes in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr J. 2009;56:601–8. doi: 10.1507/endocrj.k08e-312. [DOI] [PubMed] [Google Scholar]
- 34.Nebesio TD, Eugster EA. Observation of hypertension in children with 21-hydroxylase deficiency: A preliminary report. Endocrine. 2006;30:279–82. doi: 10.1007/s12020-006-0005-4. [DOI] [PubMed] [Google Scholar]
- 35.de Silva KS, Kanumakala S, Brown JJ, Jones CL, Warne GL. 24-hour ambulatory blood pressure profile in patients with congenital adrenal hyperplasia – A preliminary report. J Pediatr Endocrinol Metab. 2004;17:1089–95. doi: 10.1515/jpem.2004.17.8.1089. [DOI] [PubMed] [Google Scholar]
- 36.Völkl TM, Simm D, Dötsch J, Rascher W, Dörr HG. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91:4888–95. doi: 10.1210/jc.2006-1069. [DOI] [PubMed] [Google Scholar]
- 37.Hoepffner W, Herrmann A, Willgerodt H, Keller E. Blood pressure in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Pediatr Endocrinol Metab. 2006;19:705–11. doi: 10.1515/jpem.2006.19.5.705. [DOI] [PubMed] [Google Scholar]
- 38.Gorostidi M, Sobrino J, Segura J, Sierra C, de la Sierra A, Hernández del Rey R, et al. Ambulatory blood pressure monitoring in hypertensive patients with high cardiovascular risk: A cross-sectional analysis of a 20,000-patient database in Spain. J Hypertens. 2007;25:977–84. doi: 10.1097/HJH.0b013e32809874a2. [DOI] [PubMed] [Google Scholar]
- 39.Sartorato P, Zulian E, Benedini S, Mariniello B, Schiavi F, Bilora F, et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:1015–8. doi: 10.1210/jc.2006-1711. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 41.Ballantyne CM, Nambi V. Markers of inflammation and their clinical significance. Atheroscler Suppl. 2005;6:21–9. doi: 10.1016/j.atherosclerosissup.2005.02.005. [DOI] [PubMed] [Google Scholar]


