Table 2.
Pyrimido-β-carbolines based on 9.
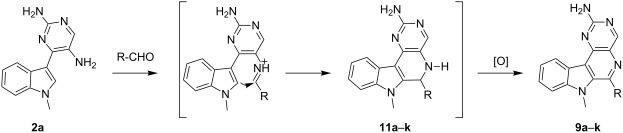 | ||||||
| Entry | Substrate | R-CHO | Pictet–Spengler products 9, R | Yield (%)a | tR (min)b | |
| 1 | 2a | 4-Cl-C6H4-CHO | 9a | 4-Cl-C6H4 | 84 | 14.92 |
| 2 | 2a | 4-OEt-C6H4-CHO | 9b | 4-OEt-C6H4 | 82 | 14.14 |
| 3 | 2a | 4-OMe-C6H4-CHO | 9c | 4-OMe-C6H4 | 78 | 13.25 |
| 4 | 2a | 4-Br-C6H4-CHO | 9d | 4-Br-C6H4 | 85 | 13.90 |
| 5 | 2a | 3,4-diCl-C6H-CHO | 9e | 3,4-diCl-C6H3 | 80 | 14.50 |
| 6 | 2c | 4-Br-C6H4-CHO | 9f | 4-Br-C6H4 | 72 | 15.89 |
| 7 | 2c | 4-NO2-C6H4-CHO | 9g | 4-NO2-C6H4-CHO | 76 | 15.82 |
| 8 | 2c | 4-OMe-C6H4-CHO | 9h | 4-OMe-C6H4-CHO | 79 | 13.45 |
| 9 | 2c | 3,4-diOMe-C6H3-CHO | 9i | 3,4-diOMe-C6H3 | 81 | 12.95 |
| 10 | 2c | 4-Cl-C6H4-CHO | 9j | 4-Cl-C6H4 | 86 | 14.82 |
| 11 | 2c | 2-Cl-C6H4-CHO | 9k | 2-Cl-C6H4 | 78 | 14.37 |
aIsolated yield. bRetention time on HPLC (C18 reversed-phase column; 150 mm × 4.8 mm; 5 µm) with a linear gradient of 0–100% CH3CN in water over 30 min. Flow rate of 1.0 mL/min and UV detection at 220/254 nm.
