Table 1.
Reaction optimization for coupling of 1e with benzamide (2a) under various conditionsa.
 | |||||
| Entry | Pd catalyst (5 mol %) | Ln | Base | Time (h) | Yield (%)b |
| 1 | Pd(OAc)2c | L1 | Cs2CO3 | 2 | 95d |
| 2 | Pd2(dba)3 | L1 | Cs2CO3 | 5 | 90 |
| 3 | Pd2(dba)3 | L2 | Cs2CO3 | 24 | 40 |
| 4 | Pd2(dba)3 | L3 | Cs2CO3 | 24 | 40 |
| 5 | Pd2(dba)3 | L4 | Cs2CO3 | 24 | 0 |
| 6 | Pd(OAc)2 | L2 | Cs2CO3 | 24 | 45 |
| 7 | Pd(OAc)2 | L3 | Cs2CO3 | 24 | 61 |
| 8 | Pd(OAc)2 | L1 | K2CO3 | 4 | 83 |
| 9 | Pd(OAc)2 | L1 | K3PO4 | 3 | 88 |
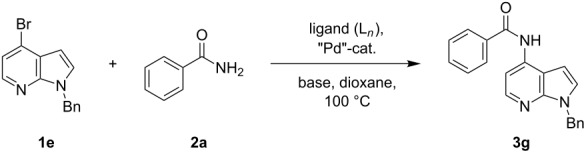
aReactions of 1-benzyl-4-bromo-1H-pyrrolo[2,3-b]-pyridine (1e) (1.0 mmol) with benzamide (2a) (1.2 mmol) were performed in a sealed Schlenk tube at 100 °C in dioxane (2 mL) by using Pd catalyst (5 mol %), ligand (10 mol %) and base (1.5 mmol). bYields reported are isolated yields. cNo reaction occurred without palladium catalyst. dNo reaction occurred at room temperature.
