Table 3.
Optimization of the coupling reaction of 4-bromo-1-ethyl-1H-pyrrolo[2,3-b]pyridine (1d) with phenylmethanamine (4a).a
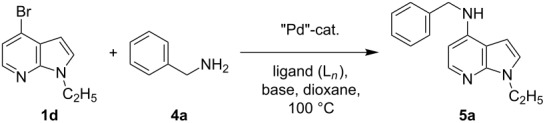 | |||||
| Entry | Pd-catalyst (5 mol %) | Ln | Base | Time (h) | Yield (%)b |
| 1 | Pd2(dba)3 | L1 | Cs2CO3 | 1 | 93 |
| 2 | Pd2(dba)3 | L1 | K2CO3 | 3 | 85 |
| 3 | Pd2(dba)3 | L2 | Cs2CO3 | 6 | 60 |
| 4 | Pd2(dba)3 | L3 | Cs2CO3 | 6 | 62 |
| 5 | Pd2(dba)3 | L4 | Cs2CO3 | 24 | 0 |
| 6 | Pd(OAc)2 | L1 | Cs2CO3 | 24 | 20 |
| 7 | Pd(OAc)2 | L1 | K2CO3 | 24 | 15 |
| 8 | Pd(OAc)2 | L1 | NaOt-Bu | 24 | 23 |
| 9 | Pd(OAc)2 | L1 | K3PO4 | 24 | 20 |
| 10 | Pd(OAc)2 | L2 | Cs2CO3 | 24 | 18 |
| 11 | Pd(OAc)2 | L3 | Cs2CO3 | 24 | 17 |
| 12 | Pd(OAc)2 | L4 | Cs2CO3 | 24 | 0 |
aReactions of 1-ethyl-4-bromo-1H-pyrrolo[2,3-b]-pyridine (1d) (1.0 mmol) with phenylmethanamine (4a) (1.2 mmol) were performed in a sealed Schlenk tube at 100 °C in dioxane (2 mL) by using Pd catalyst (5 mol %), ligand (10 mol %) and base (1.5 mmol). bYields reported are isolated yield.
