Table 4.
C–N-bond-formation cross coupling of N-protected 4-bromo-7-azaindoles 1 with amines 4.
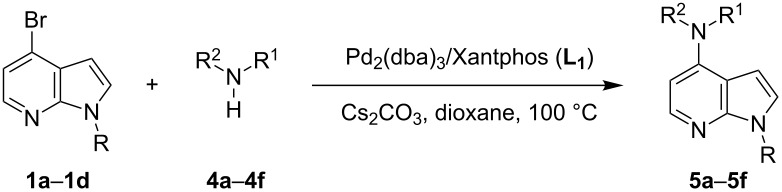 | |||||
| Entry | 7-Azaindole 1 | Amine 4 | Product 5a | Time (h) | Yield (%)b |
| 1 |
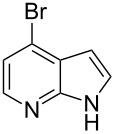 1a |
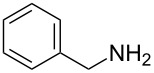 4a |
– | 24 | NRc |
| 2 |
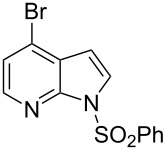 1b |
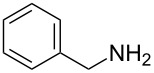 4a |
– | 3 | 0d |
| 3 |
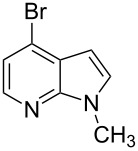 1c |
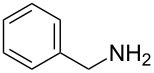 4a |
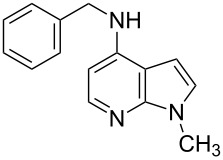 5a |
2.5 | 92 |
| 4 |
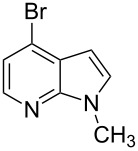 1c |
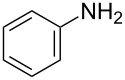 4b |
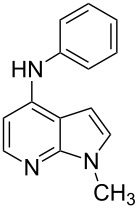 5b |
3 | 91 |
| 5 |
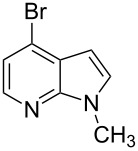 1c |
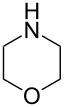 4c |
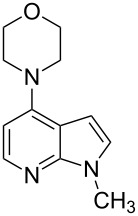 5c |
3 | 88 |
| 6 |
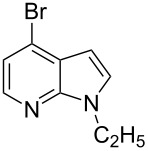 1d |
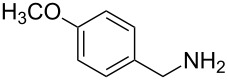 4d |
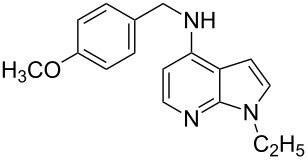 5d |
2.5 | 93 |
| 7 |
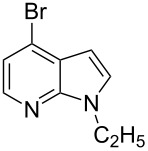 1d |
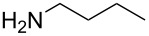 4e |
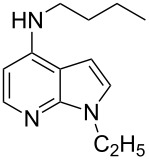 5e |
2.5 | 90 |
| 8 |
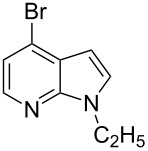 1d |
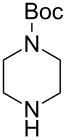 4f |
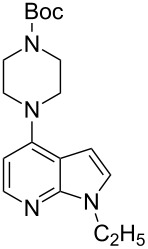 5f |
3 | 94 |
aAll reactions were carried out at 100 °C by using N-substituted 4-bromo-azaindoles 1 (1.0 mmol), amines (1.2 mmol), Cs2CO3 (1.5 mmol), Pd2(dba)3 (5 mol %), Xantphos (10 mol %), and 2 mL of dioxane. bYields reported are isolated yields. cNR: no reaction. dDesulfonation reaction takes place.
