Table 6.
C–N-bond-formation cross coupling of N-protected 4-bromo-7-azaindoles 1 with amino acid (esters) 6.
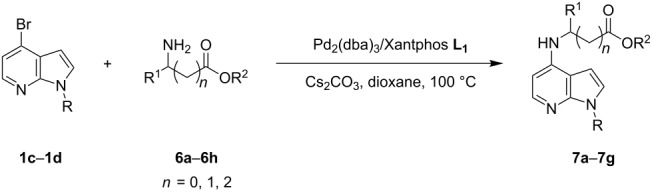 | ||||||
| Entry | 7-Azaindole 1 | Amino acid (ester) 6 | Product 7a | Time (h) | Yield (%)b | ee (%)d |
| 1 |
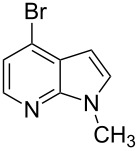 1c |
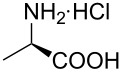 6a |
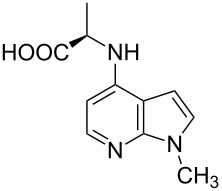 7a |
2 | traces | – |
| 2 |
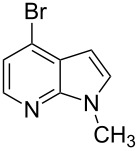 1c |
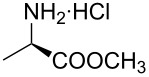 6b |
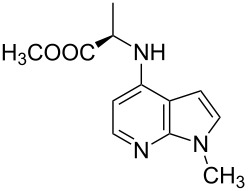 7b |
2 | 70 | 98.79 |
| 3 |
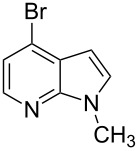 1c |
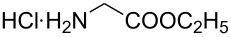 6c |
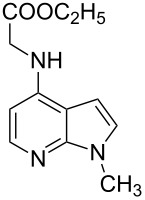 7c |
2 | 72 | – |
| 4 |
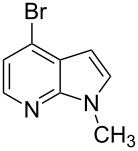 1c |
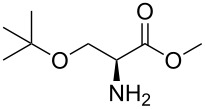 6d |
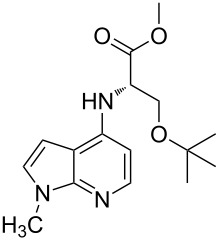 7d |
3 | 65 | 95.48 |
| 5 |
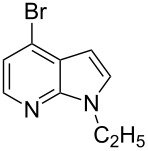 1d |
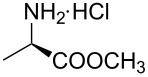 6b |
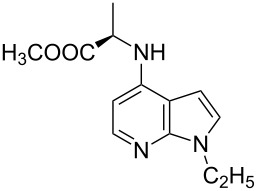 7e |
2 | 71 | 98.91 |
| 6 |
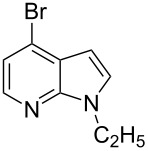 1d |
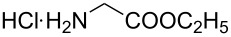 6c |
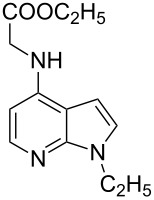 7f |
2.1 | 72 | – |
| 7 |
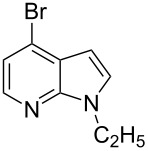 1d |
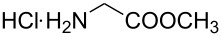 6e |
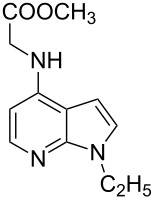 7g |
2 | 70 | – |
| 8 |
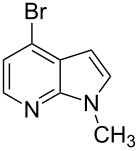 1c |
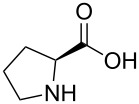 6f |
– | 5 | 0 | – |
| 9 |
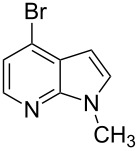 1c |
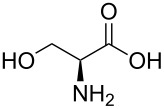 6g |
– | 5 | 0 | – |
| 10 |
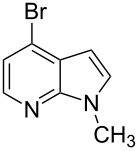 1c |
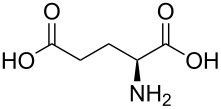 6h |
– | 5 | 0 | – |
aAll reactions were carried out at 100 °C. N-substituted 4-bromo-azaindoles 1c or 1d (1.0 mmol), amino acid (esters) (1.2 mmol), Cs2CO3 (3.0 mmol), Pd2(dba)3 (5 mol %) and Xantphos (10 mol %) were used for all the reactions. bYields reported are isolated yields. cDesulfonation reaction takes place. dee was determined by chiral HPLC.
