Table 7.
Optimization of the coupling reaction of N-methyl-4-bromo-7-azaindole (1c) with m-cresol (8a).a
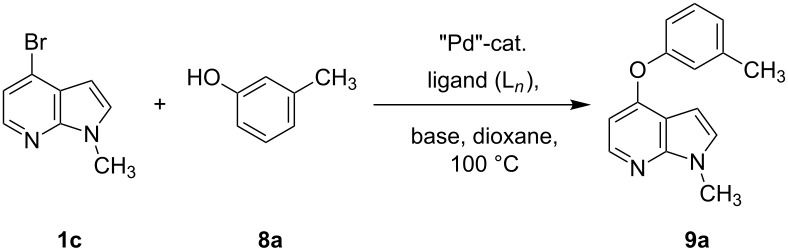 | ||||||
| Entry | Pd catalyst (5 mol %) | Ln | Base | Solvent | Time (h) | Yield (%)b |
| 1 | Pd(OAc)2 | L1 | Cs2CO3 | dioxane | 5 | 20 |
| 2 | Pd(OAc)2 | L1 | K2CO3 | dioxane | 3 | 30 |
| 3 | Pd(OAc)2 | L1 | K2CO3 | dioxane | 10 | 70 |
| 4 | Pd(OAc)2 | L1 | K2CO3 | THF | 10 | 50 |
| 5 | Pd2(dba)3 | L1 | Cs2CO3 | dioxane | 3 | 10 |
| 6 | Pd2(dba)3 | L1 | K2CO3 | dioxane | 10 | 32 |
| 7 | Pd(OAc)2 | L2 | Cs2CO3 | dioxane | 24 | 45 |
| 7 | Pd(OAc)2 | L3 | Cs2CO3 | dioxane | 24 | 61 |
| 9 | Pd(OAc)2 | L1 | K2CO3 | dioxane | 12 | 68 |
| 10 | Pd(OAc)2 | L1 | K2CO3 | dioxane | 24 | 65 |
aReaction conditions: N-methyl-4-bromo-7-azaindole (1c) (1.0 mmol), m-cresol (1.2 mmol), base (3.0 mmol), palladium catalyst (5 mol %), ligand (10 mol %), and 2 mL of dioxane, 100 °C, 3–24 h. bYields reported are isolated yields.
