Fig. 2.
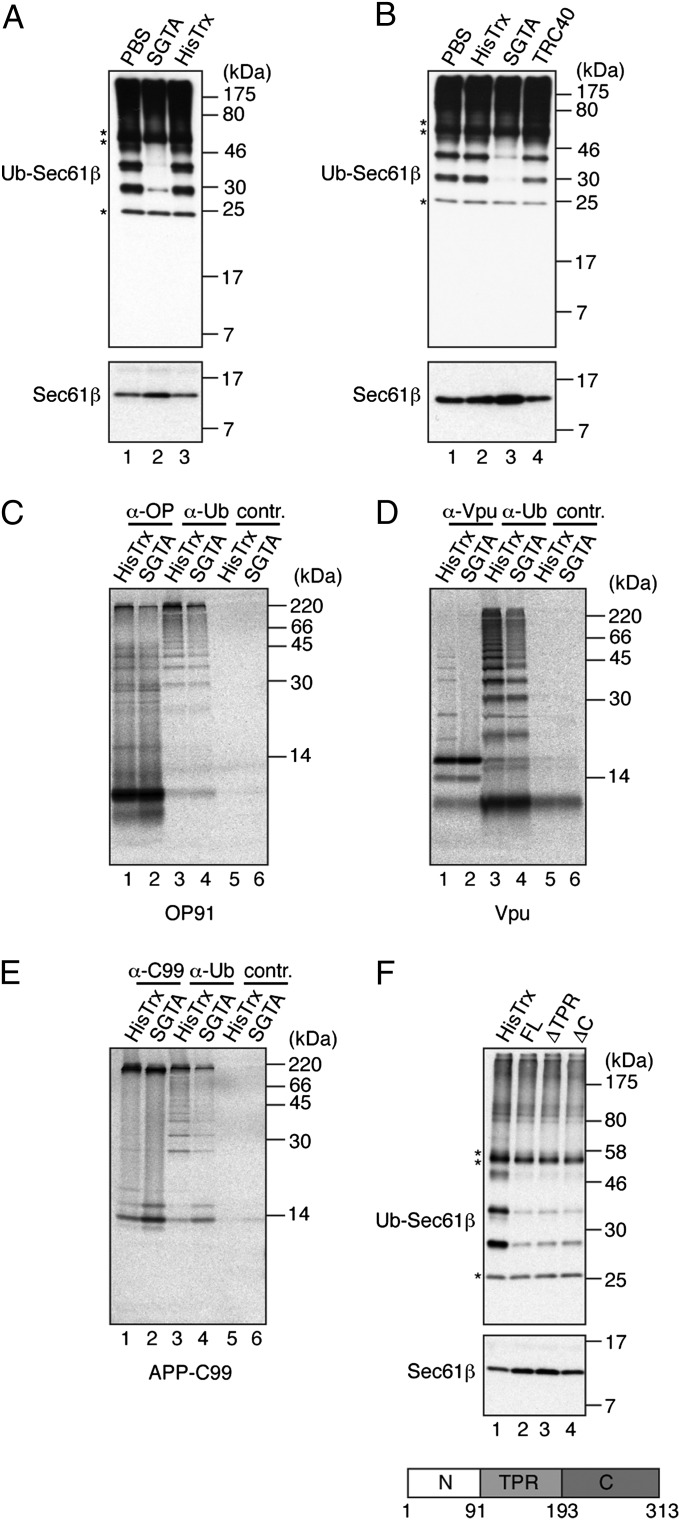
SGTA inhibits the ubiquitination of a range of hydrophobic precursors. (A) Sec61β ubiquitination reaction as for Fig. 1 (2 h) but in the presence of 4 μM HisTrx-SGTA (SGTA), HisTrx, or an equal volume of PBS. Samples were analyzed by Western blotting as for Fig. 1. (B) Specificity of the SGTA-dependent effect was confirmed by comparing Sec61β ubiquitination in the presence of TRC40. (C–E) Substrates indicated were translated in rabbit reticulocyte lysate in the presence of 2 μM HisTrx-SGTA (SGTA) or 2 μM HisTrx control, treated with NEM, and then immunoprecipitated with antibodies against the substrate (lanes 1 and 2), ubiquitin (lanes 3 and 4), or a control (lanes 5 and 6). Products were resolved by SDS/PAGE and visualized by phosphorimaging. APP-C99, C-terminal 99 amino acids of amyloid precursor protein (APP) fused with its N-terminal signal sequence; OP91, N-terminal 91 residues of bovine rhodopsin; Vpu, viral protein U of HIV (Fig. S2). (F) Sec61β ubiquitination was as for A but in the presence of 4 μM HisTrx, full-length His-S-SGTA (FL), or SGTA deletion mutants lacking the tetratricopeptide repeats (ΔTPR) or the C-terminal glutamine-rich region (ΔC). SGTA organization with domain boundaries is shown.