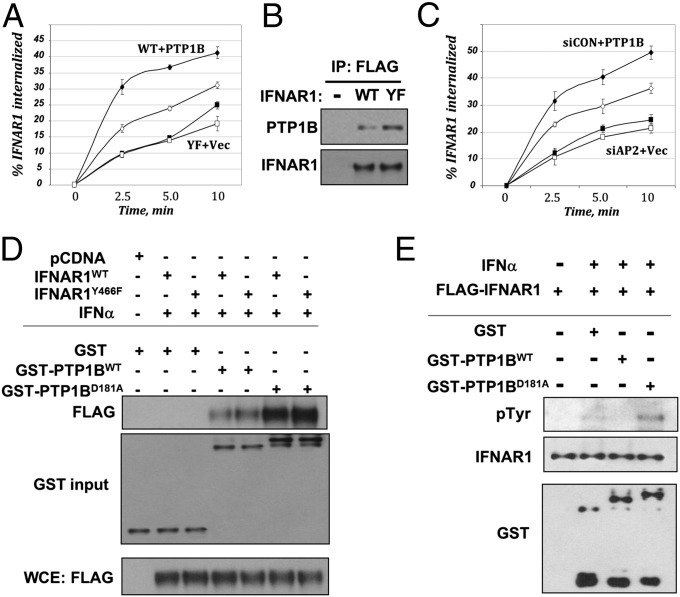
Fig. 2.
PTP1B regulates Tyr phosphorylation and protein stability of IFNAR1 in human cells. (A) Internalization of FLAG–IFNAR1 (WT, diamonds; or Y466F mutant, squares) in IFN-α–treated 293T cells that were cotransfected with empty vector (open symbols) or PTP1B (filled symbols). (B) Interaction between endogenous PTP1B and FLAG–IFNAR1 expressed in 293T cells treated with IFN-α was determined by immunoprecipitation–immunoblotting using the indicated antibodies. Loading was normalized to achieve comparable levels of FLAG–IFNAR1 proteins. (C) Internalization of endogenous IFNAR1 in IFN-α–treated 293T cells that received RNAi against AP2 (squares) or against luciferase (siCON; diamonds) and were cotransfected with either empty vector (open symbols) or PTP1B (filled symbols). (D) The interaction between bacterially produced GST-tagged PTP1B proteins (WT or D181A mutant) with FLAG-tagged IFNAR1 (WT or Y466F mutant) expressed in cells treated or not with IFN-α as indicated were analyzed by pulldown (using GSH beads) and immunoblotting. Normalized input levels of FLAG–IFNAR1 in the cell lysates used for binding experiments are also shown. (E) In vitro Tyr dephosphorylation of FLAG-tagged IFNAR1 purified from 293T cells treated or not with IFN-α upon the incubation with indicated bacterially produced GST-tagged protein (for 30 min at 37 °C) was analyzed by using the anti-phospho-Tyr antibody.