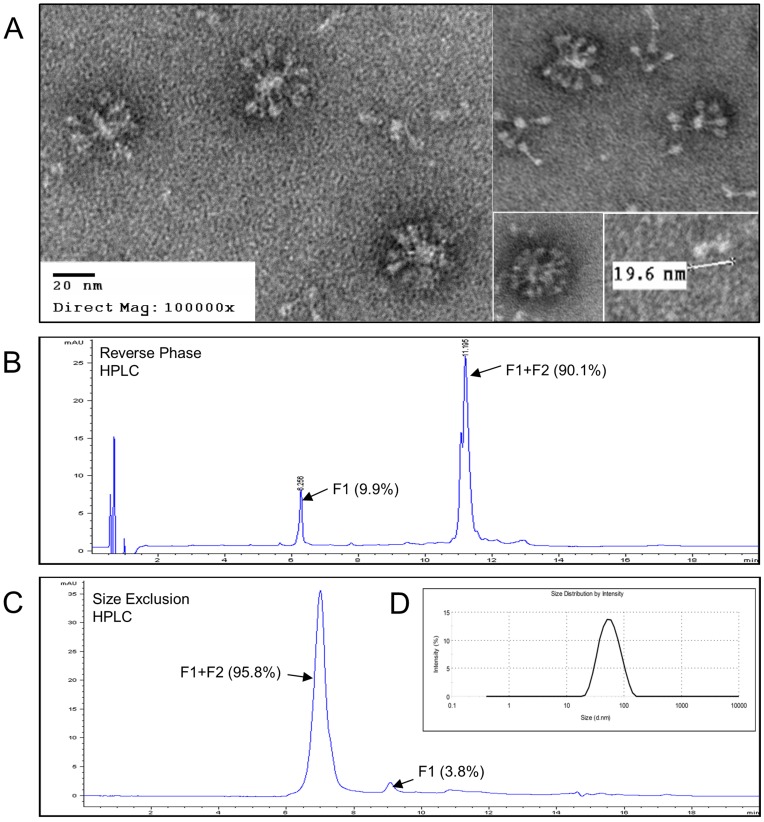
Figure 4. EM, HPLC, and DLS analysis of purified RSV F.
(A) Negative staining electronmicroscopy of purified RSV F produced in Sf9 cells with the baculovirus vector BV683. RSV F nanoparticles were adsorbed to carbon parlodion-coated copper grids and negatively stained with 1% phosphotungstic acid. Bar represent 20 nm. (B) Reverse phase HPLC analysis on a PLRP-S 4000A, 2.1×150 mm column purified RSV F consisted of 90.1% F1+F2 at retention time of 11.195 min and 9.9% F1 at retention time of 6.256. (C) RSV vaccine particle size estimation by size exclusion HPLC RSV F protein using a BEH200, 1.7 µm, 4.6×300 mm size exclusion column. Size exclusion chromatography showed the RSV F nanoparticles consist primarily of F0 (covalently linked F1 and F2) with a low level of free F1 subunits. F0 (F1+F2) peak is 95.8% and F1 peak is 3.8% of the total peak area. (D) Dynamic light scattering of purified RSV F showing the distribution (%) of the intensity of light scattered by the particles using a detector placed at an angle 173°. Relative light intensity (%) was plotted relative to the particle diameter in nanometers (d.nm) of RSV F.