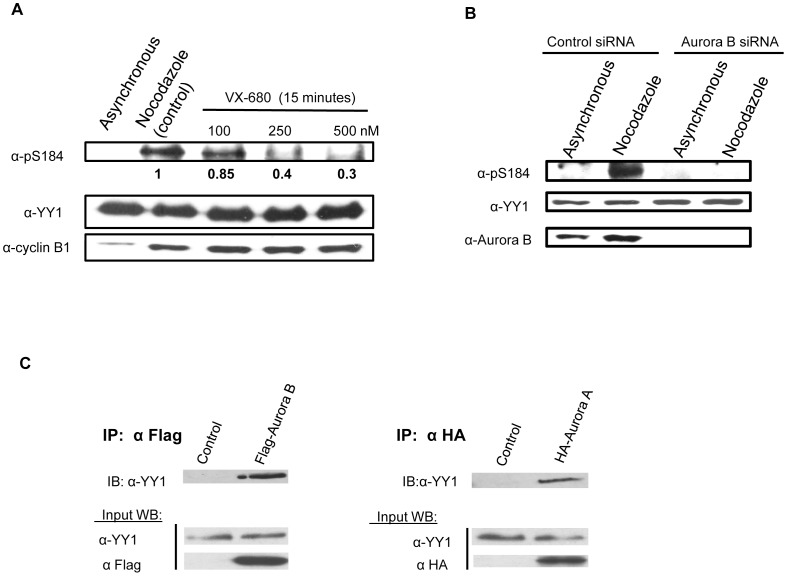
Figure 4. Aurora B phosphorylates YY1 at serine 184 in vivo.
(A) HEK293 cells were synchronized in mitosis with nocodazole block for 17 hours. After the block, the cells were treated with the Aurora inhibitor VX-680, with the indicated concentrations for 15 minutes. Cell lysates were prepared, followed by Western blot. The blot was probed with anti-pS184 antibody with relative levels indicated below, then stripped and reprobed with anti-YY1 antibody followed by anti-cyclin B1antibody to show proper mitotic synchrony. (B) HEK293 cells were plated, cultured overnight, and then transfected with 20 nm control scrambled siRNA or Aurora B siRNA. After 48 hours of knockdown, the cells were lysed, and extracts were analyzed by Western blotting. The blot was probed with anti-pS184 antibody then stripped and reprobed with anti-YY1 antibody followed by anti-Aurora B antibody. (C) Co-immunoprecipitation of YY1 with Aurora A and Aurora B from HEK293 cells transiently transfected with HA-Aurora A, Flag-Aurora B and no transfection (control) followed by nocodazole block. Aurora A was immunoprecipitated using anti-HA antibody and Aurora B was immunoprecipitated using anti-Flag mouse MAb cross-linked to resin beads. Non-transfected cells were also immunprecipitated using anti-HA antibody and anti-Flag mouse MAb cross-linked to resin beads, which were used as a control for the specificity of the immunoprecipitation. Western blot analysis was performed on the immunoprecipitated samples and probed with anti-YY1 antibody. Input samples were probed with anti-YY1 antibody, anti-HA antibody and anti-Flag antibody.