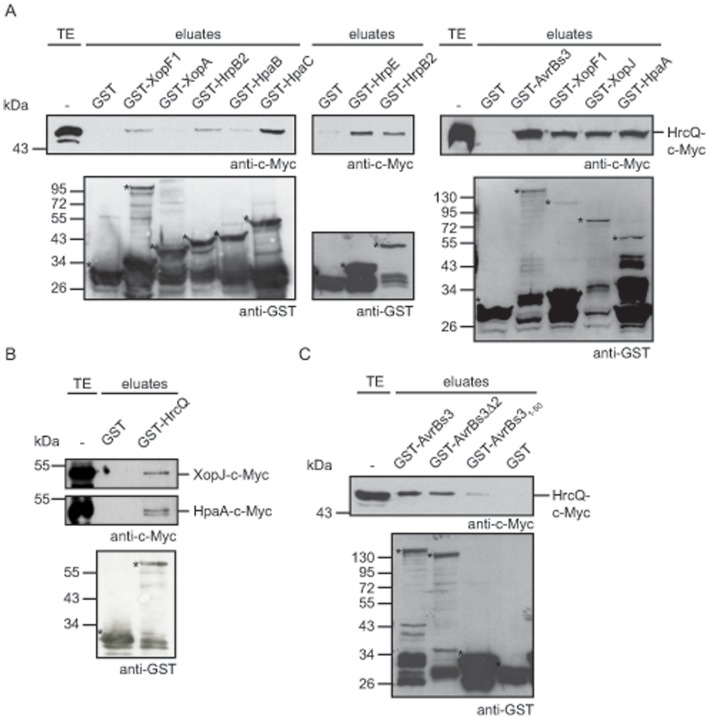
Figure 6. HrcQ provides a docking site for early and late T3S substrates.
(A) HrcQ interacts with T3S substrates and the T3S4 protein HpaC. GST, GST-XopF1, GST-XopA, GST-HrpB2, GST-HpaB, GST-HpaC, GST-HrpE, GST-AvrBs3, GST-XopJ and GST-HpaA were immobilized on glutathione sepharose and incubated with bacterial lysates containing HrcQ-c-Myc. Total cell extracts (TE) and eluted proteins (eluates) were analyzed by immunoblotting using c-Myc epitope- and GST-specific antibodies. GST and GST fusion proteins are marked with asterisks, additional bands correspond to degradation products. (B) HrcQ interacts with XopJ and HpaA. GST and GST-HrcQ were immobilized on glutathione sepharose and incubated with bacterial lysates containing XopJ-c-Myc and HpaA-c-Myc, respectively. TE and eluates were analyzed as is described in panel A. One representative blot incubated with GST-specific antibodies is shown. (C) The N-terminal region of AvrBs3 is dispensable for the interaction with HrcQ. GST, GST-AvrBs3, GST-AvrBs3Δ2 and GST-AvrBs31-50 were immobilized on glutathione sepharose and incubated with a bacterial lysate containing HrcQ-c-Myc. TE and eluates were analyzed as is described in panel A.