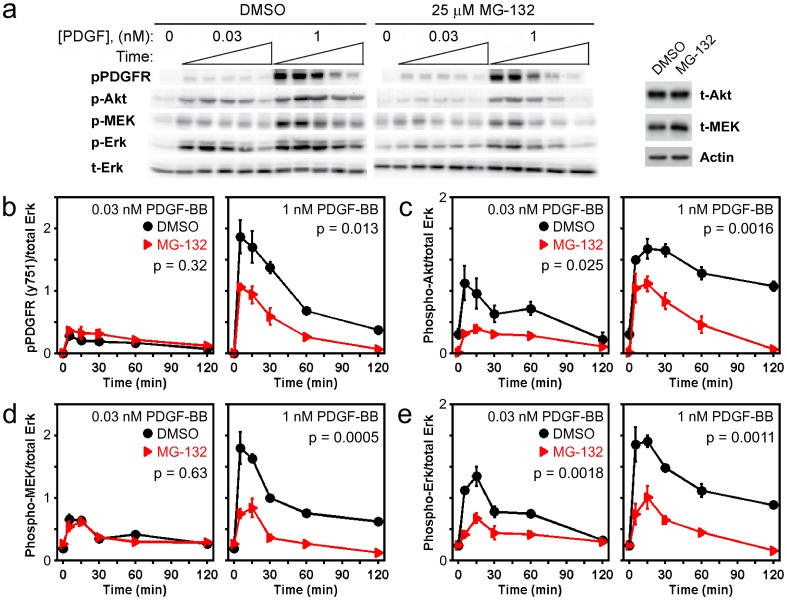
Figure 1. MG132 treatment depresses PDGF-stimulated phosphorylation of ERK and also reduces the activation of upstream signaling components.
a) Immunoblot results, representative of 3 independent experiments, showing the phosphorylation kinetics of PDGF β-receptor Tyr751 (pPDGFR), Akt1/2/3 Ser473 (pAkt), MEK1/2 Ser217/Ser221 (pMEK), and ERK1/2 Thr202/Tyr204 (pERK) in cells pretreated with either DMSO or 25 µM MG132 for 6 h and then stimulated with the indicated concentration of PDGF-BB. Stimulation times are 5, 15, 30, 60, and 120 minutes. Total ERK1/2 (tERK) serves as a loading control. For each antigen, the DMSO and MG132 bands are cropped from the same gel. At right it is shown that total Akt (tAkt) protein expression is not affected by MG132 treatment, whereas total MEK1/2 (tMEK) is only modestly increased in MG132-treated cells, relative to β-actin loading control. b-e) Quantification of the phosphorylation kinetics represented in a. Each readout is normalized by total ERK and expressed as mean ± s.e.m. (n = 3): b, pPDGFR; c, pAkt; d, pMEK; e, pERK. The indicated p value for each time course is from two-way ANOVA analysis comparing MG132-treated and control measurements.