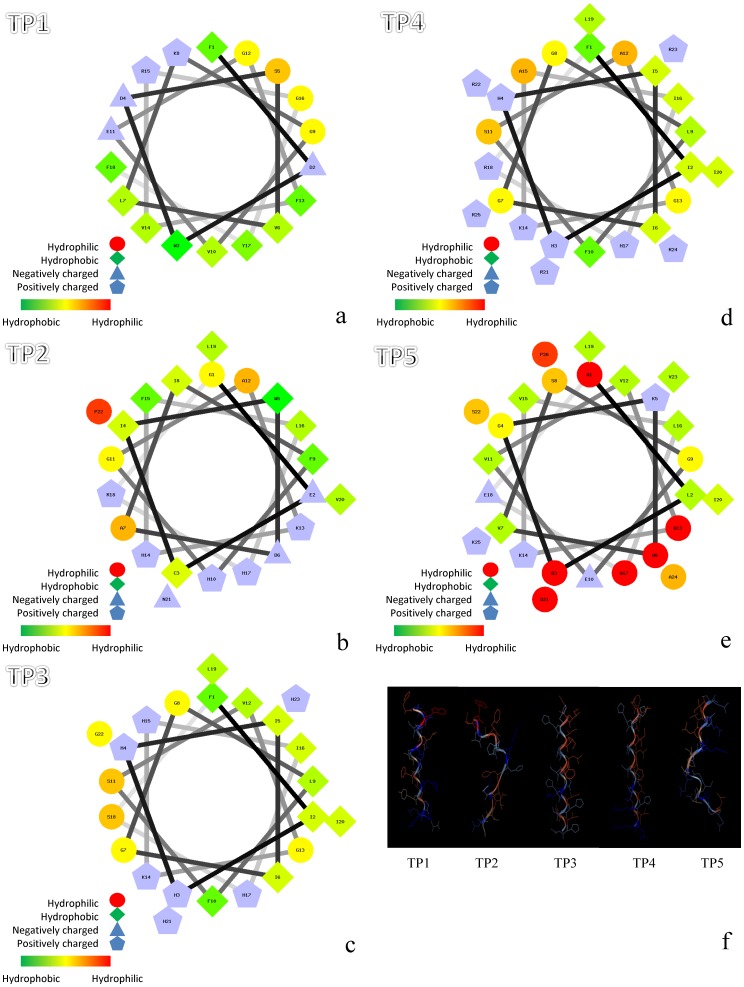
Figure 3. Amphipathic α-helical structures and three-dimensional structures of five different Nile tilapia piscidins.
A Schiffer-Edmunson plot of (a) Nile tilapia piscidin 1 (TP1), (b) -2, (c) -3, (d) -4, and (e) -5 was produced with the PEPWHEEL program. Amino acid residues are successively numbered and connect each amino acid following the sequence with lines that represent its relative position along the helix. Red indicates hydrophilic, green indicates hydrophobic, blue triangles indicate negatively charged, and blue pentagons indicate positively charged residues. Structural models of (f) TP1∼5. Pictures were generated with the Viewerlite program (Accelrys, San Diego, CA, USA).