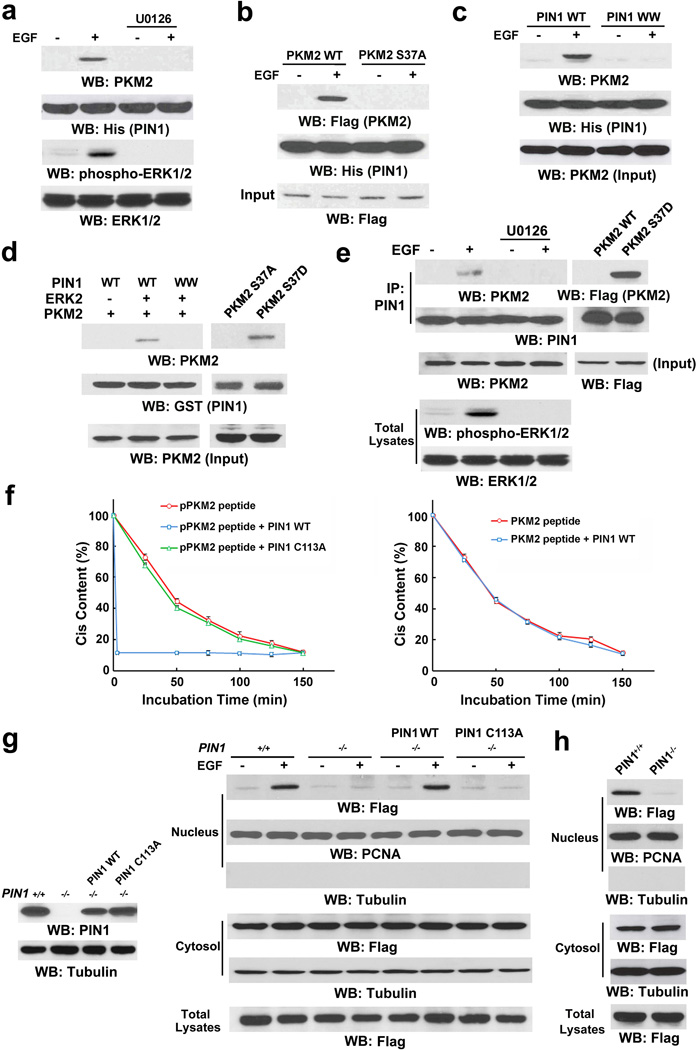
Figure 3. PKM2 S37 phosphorylation recruits PIN1.
Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies.
a, His-PIN1 immobilized on nickel agarose beads was incubated with lysate of U87/EGFR cells treated with or without U0126 (20 µM) for 30 min before EGF (100 ng/ml) stimulation for 30 min.
b, U87/EGFR cells transiently expressing WT Flag-PKM2 or Flag-PKM2 S37A were treated with or without EGF (100 ng/ml) for 30 min. His-PIN1 immobilized on nickel agarose beads was incubated with the cell lysates.
c, His-PIN1 or His-PIN1 WW domain mutant (with substitutions at W11A, W34A, R14A, and R17A) immobilized on nickel agarose beads was incubated with lysate of U87/EGFR cells treated with or without EGF (100 ng/ml) for 30 min.
d, Purified GST-PIN1 on glutathione agarose beads was mixed with purified His-PKM2 with or without purified active ERK2 (left panel) or incubated with purified His-PKM2 S37A or His-PKM2 S37D mutant (right panel).
e, PIN1 was immunoprecipitated from U87/EGFR cells pretreated with or without U0126 (20 µM) for 30 min before EGF (100 ng/ml) stimulation for 30 min (left panel) or from U87/EGFR cells expressing Flag-PKM2 or Flag-PKM2 S37D (right panel).
f, Cis-trans isomerization assays were performed by mixing synthesized phosphorylated or nonphosphorylated oligopeptide of PKM2 containing the S37P motif with purified WT GST-PIN1 or GST-PIN1 C113A mutant. Data represent the means ± SD of three independent experiments.
g, PIN1-/- cells were reconstituted to express the indicated PIN1 proteins (left panel). The total cell lysates and nuclear fractions were prepared from the indicated cells treated with or without EGF (100 ng/ml) for 6 h (right panel).
h, Total cell lysates and nuclear fractions of PIN1-/- cells or PIN1-/- cells expressing Flag-PKM2 S37D were prepared.