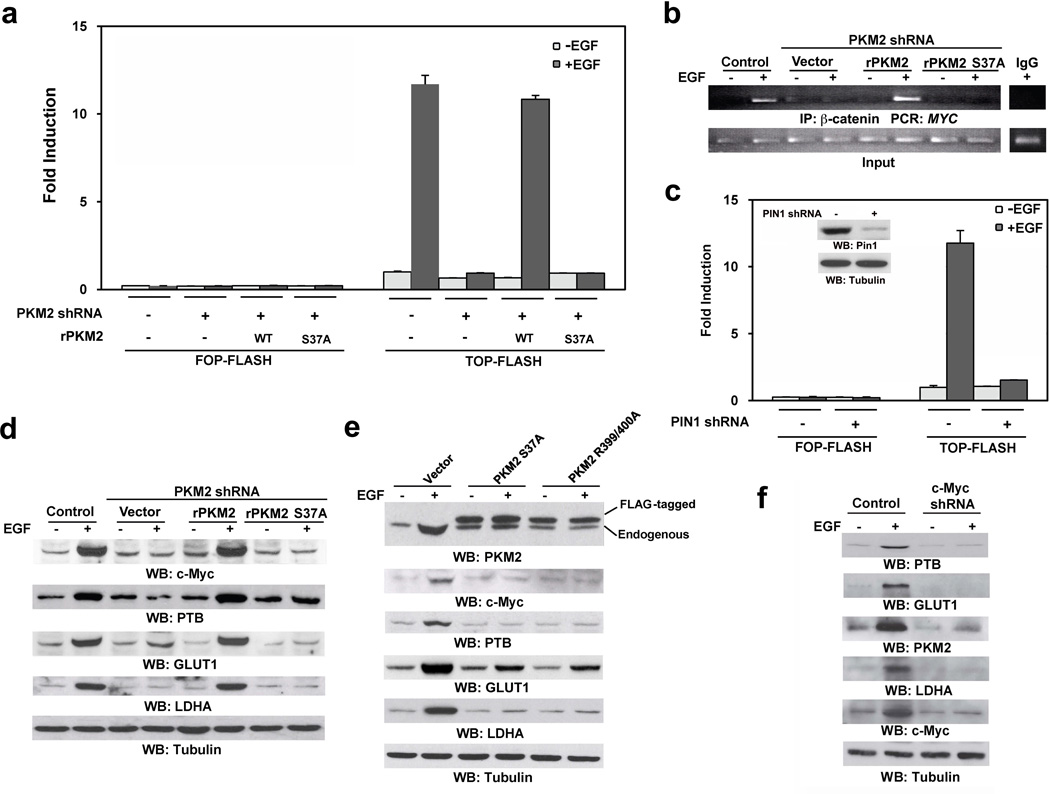
Figure 5. Nuclear PKM2 regulates glycolytic gene expression.
Immunoblotting analyses were performed with the indicated antibodies.
a, c, U87/EGFR cells with or without PKM2 depletion and reconstituted expression of the indicated PKM2 proteins (a) or U87/EGFR cells with or without PIN1 depletion (c) were transfected with either TOP-FLASH or FOP-FLASH (control vector), which was followed by EGF (100 ng/ml) treatment for 12 h. The relative levels of luciferase activity were normalized to the levels of untreated cells and to the levels of luciferase activity of the Renilla control plasmid. Data represent the means ± SD of three independent experiments.
b, U87/EGFR cells with or without PKM2 depletion and reconstituted expression of the indicated PKM2 proteins were treated with or without EGF (100 ng/ml) for 12 h. ChIP assay was performed with an anti-β-catenin antibody for immunoprecipitation followed by PCR with MYC promoter–specific primers.
d, U87/EGFR cells with or without PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 S37A mutant were treated with or without EGF (100 ng/ml) for 24 h.
e, U87/EGFR cells expressing Flag-PKM2 S37A or Flag-PKM2 R399/400A were treated with or without EGF (100 ng/ml) for 24 h.
f, U87/EGFR cells with or without c-Myc depletion were treated with or without EGF (100 ng/ml) for 24 h.