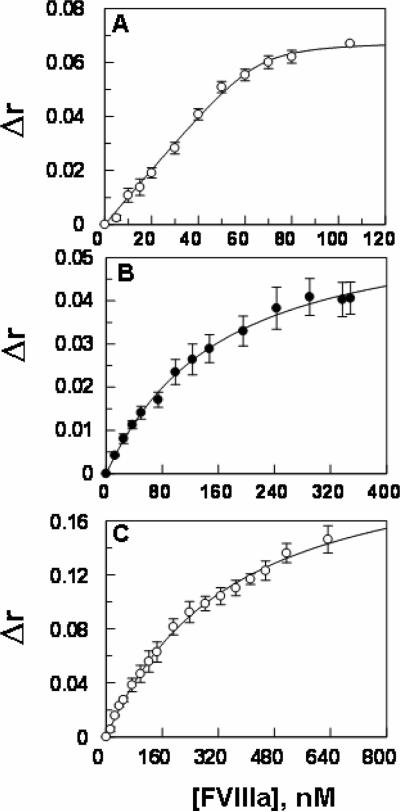
Figure 5.
Enhancement in the anisotropy of the OG488-EGR labeled FIXa derivatives upon interaction with FVIIIa on PCPS vesicles. A, a fixed concentration of the OG488-EGR labeled wild-type FIXa (50 nM) was titrated with increasing concentrations of FVIIIa (0.25–120 nM) on PCPS vesicles (50 μM) in TBS containing 0.1% PEG 8000 and 5 mM Ca2+ at 25 °C. KD (1.5 ± 1.1 nM, n =3) of FIXa for interaction with FVIIIa was calculated from the saturable changes in the anisotropy (Δr) of the labeled protein according to a quadratic binding equation as described under “Materials and methods”. B, the same as panel A except that OG488-EGR labeled FIXa-desEGF1 (50 nM) was used in the FVIIIa titration yielding a KD of (135.4 ± 13.7 nM, n =3) for the interaction of the mutant protease with the cofactor. C, the same as above except that OG488-EGR labeled GD-FIXa (50 nM) was used in the FVIIIa titration yielding a KD of (308.6 ± 22.4 nM, n =3) for the interaction of the mutant protease with the cofactor.