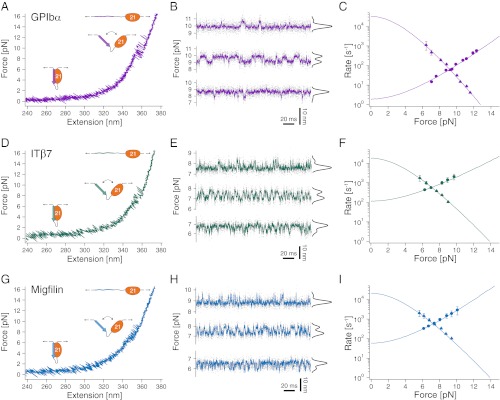
Fig. 2.
Interaction of filamin with tethered peptides. (A) Force extension trace of the interaction between the target peptide of GPIbα and FLNa21 (GPIbα-FLNa21). At forces around 10 pN, the target peptide rapidly fluctuates between bound and unbound conformations and stays permanently unbound at higher loads (>12 pN). (B) Equilibrium traces obtained at three different biasing forces. At high loads (top trace), the probability (black histogram) is shifted to the unbound state, whereas at decreasing loads, the bound state becomes more and more populated (bottom two traces). (C) Opening (circles) and closing (triangles) rates as a function of force. The solid line is an extrapolation of the rates to zero-load taking into account the compliance of all mechanical elements in the construct (SI Materials and Methods). (D) Force extension trace of the interaction between the target peptide of β7-integrin and FLNa21 (ITβ7-FLNa21). (E) Equilibrium traces of ITβ7-FLNa21 obtained at three different biasing forces (compare B). (F) Opening (circles) and closing (triangles) rates as a function of force (compare C). (G) Force extension trace of the interaction between the target peptide of migfilin and FLNa21 (Mig-FLNa21). (H) Equilibrium traces of Mig-FLNa21 obtained at three different biasing forces (compare B and E). (I) Opening (circles) and closing (triangles) rates as a function of force (compare C and F).