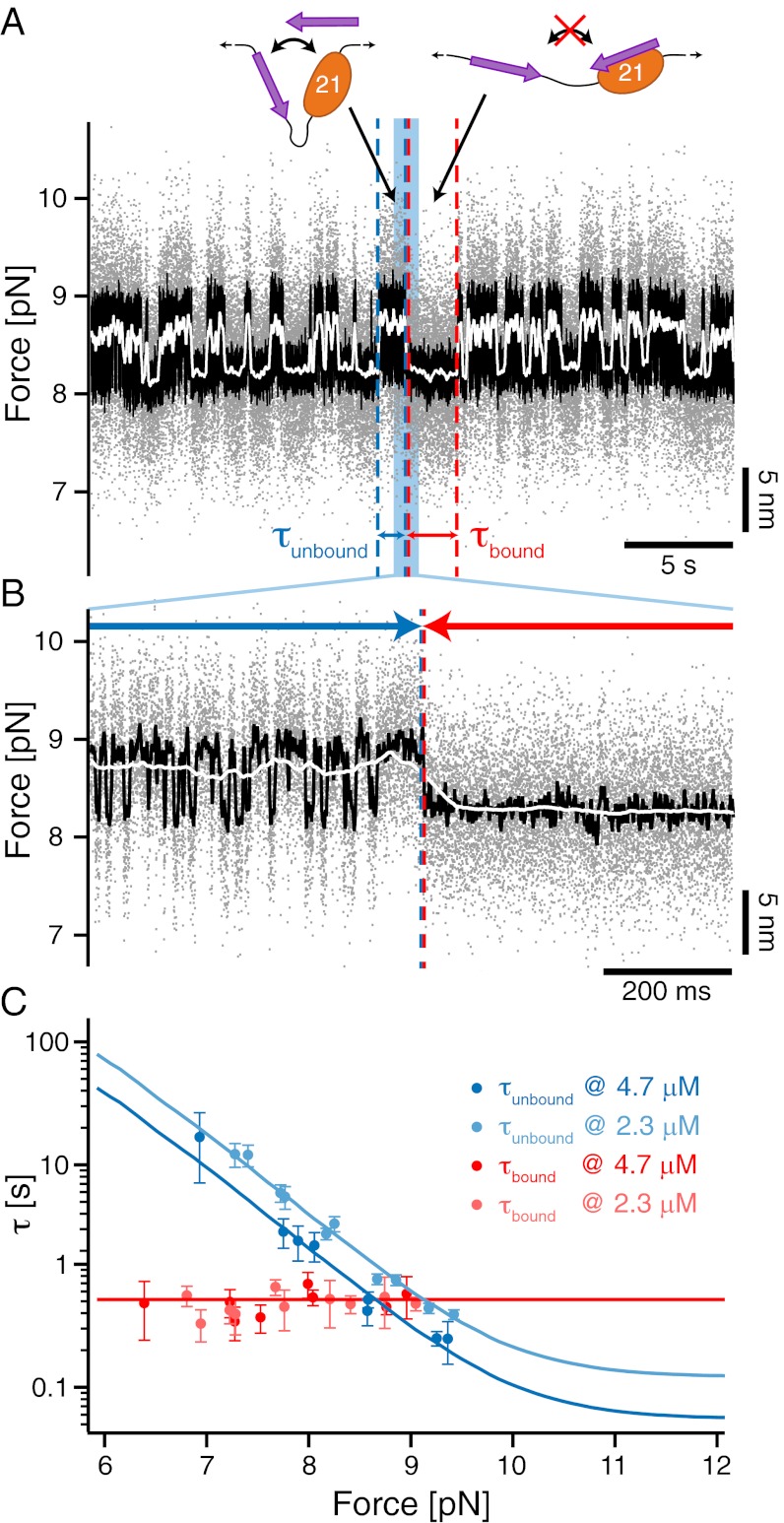
Fig. 3.
Single-molecule mechanical competition assay to study peptide binding from solution. (A) Time traces of opening and closing of the GPIbα-FLNa21 construct held at a force bias of 8.5 pN in the presence of 2.3 μM GPIbα peptide in solution. The colored traces correspond to the 20-kHz data (gray), moving average filtered with 2.5 ms (black) and 50 ms (white) time window. Apparent high-SD regions with lifetimes 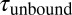 in the black and gray traces are interrupted by low-SD regions with lifetimes
in the black and gray traces are interrupted by low-SD regions with lifetimes 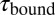 . (B) A zoom into the blue region shows the transition between a high-SD (rapid opening and closing cycles of the tethered construct) and low-SD region (blocked fluctuations due to competitive peptide binding from solution). (C) Dependence of the bound and unbound lifetimes as a function of applied force and solution concentration. As expected for binding from solution,
. (B) A zoom into the blue region shows the transition between a high-SD (rapid opening and closing cycles of the tethered construct) and low-SD region (blocked fluctuations due to competitive peptide binding from solution). (C) Dependence of the bound and unbound lifetimes as a function of applied force and solution concentration. As expected for binding from solution, 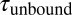 depends on the opening probability of the tethered construct and hence the applied force, as well as the solution concentration, whereas
depends on the opening probability of the tethered construct and hence the applied force, as well as the solution concentration, whereas 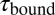 is independent (see text and SI Materials and Methods).
is independent (see text and SI Materials and Methods).