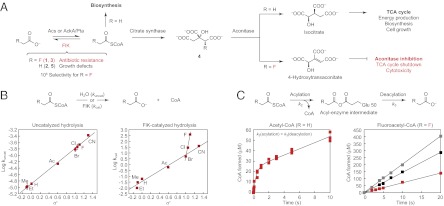
Fig. 1.
FlK selectivity for fluoroacetyl-CoA over acetyl-CoA is derived from a change in the rate-limiting step in thioester hydrolysis. (A) FlK catalyzes the hydrolysis of fluoroacetyl-CoA, which reverses the lethal synthesis of an irreversible aconitase inhibitor. (B) Taft free-energy relationship analysis of uncatalyzed (Left) and FlK-catalyzed (Right) acyl-CoA hydrolysis. Pseudo-first-order rate constants were determined by linear fitting (for uncatalyzed reactions) or by fitting a plot of initial rate versus substrate concentration to the Michaelis-Menten equation (for FlK-catalyzed reactions). Ac, acetyl; Et, ethyl; Me, methyl. (C) Pre-steady-state kinetics of FlK-catalyzed acetyl-CoA (Left, 25 μM FlK) and fluoroacetyl-CoA hydrolysis (Right; red squares, 25 μM FlK; black squares, 50 μM FlK; grey squares, 75 μM FlK).