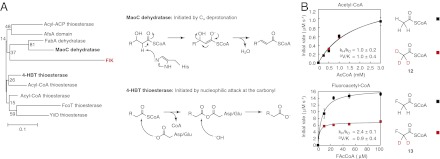
Fig. 2.
Breakdown of the fluoroacetyl-enzyme intermediate is accelerated by Cα-deprotonation. (A) Structure-based sequence alignment and phylogenetic analysis of the hotdog superfamily indicate that FlK shares functional features with both the 4-HBT thioesterases, which use an enzyme anhydride intermediate in hydrolysis, and the MaoC dehydratases, which initiate dehydration by Cα deprotonation using a catalytic histidine residue. (B) Deuteration at the α position of fluoroacetyl-CoA leads to a 2.4-fold kinetic isotope effect (KIE) on kcat for FlK-catalyzed fluoroacetyl-CoA hydrolysis, whereas deuteration at the α position of acetyl-CoA has no impact on the rate constant.