Abstract
This study aimed to estimate the risk of progression to active tuberculosis (TB) within 2 yrs after entry in newly arriving immigrants who were screened with the QuantiFERON®-TB Gold In-Tube assay (QFT-GIT; Cellestis, Carnegie, Australia).
In a case–base design, we determined the prevalence QFT-GIT-positive subjects among a representative sample of immigrants aged ≥18 yrs who arrived between April 2009 and March 2011 (the base cohort). Active TB patients (cases) within 2 yrs post-arrival in 2005, 2006 or 2007 were extracted from the Netherlands Tuberculosis Register. The risk of progression to active TB was estimated using Bayesian analyses to adjust for the sensitivity of QFT-GIT.
Among the base cohort, 20% of 1,468 immigrants were QFT-GIT positive. Stratified by TB incidence in the person's country of origin as low (<100 cases per 100,000 population), intermediate (100–199 cases per 100,000) or high (≥200 cases per 100,000), the risk of progression to active TB per 100,000 arriving immigrants if QFT-GIT positive (95% credibility interval) was 456 (95% CI 307–589), 590 (397–762) and 386 (259–499), respectively, compared with 18 (0–46), 38 (0–97) and 28 (0–71) if QFT-GIT negative.
Screening newly arriving immigrants with QFT-GIT contributes to detecting those at high risk of subsequent TB reactivation within 2 yrs after entry, which offers opportunities for prevention by targeted interventions.
Keywords: Immigration, latent tuberculosis, screening, tuberculosis
As in most western countries, the incidence of tuberculosis (TB) in the Netherlands is decreasing among the native Dutch population in contrast to first generation immigrants (persons born outside the Netherlands) [1]. In 2009, 73% of all TB cases were diagnosed in first generation immigrants [1], and this percentage is predicted to increase [2].
TB incidence in the Netherlands will not decrease further without specific interventions targeted at first generation immigrants. Entry screening for active TB by chest radiograph (CXR) is mandatory for all immigrants aged >12 yrs from primarily non-Western countries who intend to stay for >3 months [3]. Immigrants from high incidence countries (≥200 cases per 100,000) are offered voluntary follow-up screening by CXR at 6-month intervals for 2 yrs. This strategy is not effective in terms of lowering the TB incidence among first generation immigrants [4, 5]. TB in this group is largely due to reactivation of latent tuberculosis infection (LTBI) acquired in the country of origin [6]. Screening immigrants at entry for LTBI and providing those infected with prophylactic treatment is a potentially important strategy to reduce the incidence of TB [7, 8], but is not implemented in the Netherlands because of the low specificity of the tuberculin skin test (TST).
The QuantiFERON®-TB Gold In-Tube (QFT-GIT assay; Cellestis, Carnegie, Australia) identifies cellular production of interferon-γ in response to the Mycobacterium tuberculosis-specific 6-kDa early secreted antigens (ESAT-6), the 10-kDa culture filtrate protein (CFP10) and TB7.7. Previous research has shown that, compared with the TST, the QFT-GIT has a higher specificity in measuring LTBI as it is independent of previous bacille Calmette–Guérin vaccination or infection with most nontuberculous mycobacteria, two factors that are often present in an immigrant population [9].
Whether screening with QFT-GIT and treatment of LTBI in immigrants is an effective intervention depends on its discriminatory ability to identify those at high risk of progression to active TB. The risk of progression to active TB stratified by QFT-GIT result has been assessed among several populations, but never among newly arriving immigrants [10]. The objective of this study was to estimate the proportion of newly arrived immigrants with a positive QFT-GIT and to assess the risk for developing active TB within 2 yrs after entry given the QFT-GIT result at entry.
METHODS
Design
The study used a case–base design. We assessed the prevalence of QFT-GIT-positive results in a representative sample of newly arriving immigrants at seven Public Health Services (PHSs) between April 2009 and March 2011, denoted as the base cohort. This prevalence was projected on three cohorts of immigrants who were registered in the Monitoring for Screening of Immigrants (MSI programme), and were screened at arrival in 2005, 2006 or 2007 at the same seven PHSs, denoted as case source cohort. In the MSI, the PHSs register the results of screening activities. From the case source cohort, we extracted the immigrants who developed TB within 2 yrs after arrival, denoted as cases, by matching the MSI with the Netherlands Tuberculosis Register (NTR).
Data collection
For the base cohort, data was collected by trained staff at the seven PHSs located throughout the country. All immigrants aged ≥18 yrs who reported for their entry screening were invited. Asylum seekers were not included because they were not registered in the MSI. Enrolment continued until 1,500 QFT-GIT outcomes were obtained. This number was needed to determine an assumed prevalence of 30% QFT-GIT-positive subjects [11], accounting for an imprecision of 6%, and to be able to stratify analyses. Participants had a structured questionnaire and a QFT-GIT in addition to a routine CXR. Age was categorised as 18–24, 25–34 or ≥35 yrs. Country of origin was collapsed into region of origin (Europe and the Americas, the Middle East and North Africa, other Asia, Sub-Saharan Africa, and other), and TB incidence in the country of origin (low, <100 cases per 100,000; intermediate, 100–199 per 100,000; high, ≥200 per 100,000). Immunocompromised (e.g. HIV-positive) subjects and users of immunosuppressive medication were excluded for QFT-GIT testing. Cases from the base cohort and from the case source cohort who were diagnosed with TB within 6 months after the entry screening were excluded because the NTR classified these cases as being detected during entry screening. Ethical approval was obtained from the Netherlands Central Committee on Research involving Human Subjects (CCMO), the Hague, the Netherlands. All participants of the base cohort provided written informed consent.
QFT-GIT testing was performed according to manufacturer's instructions in PHSs' experienced local laboratories. Test results were interpreted according to the cut-off values provided by the manufacturer.
Statistical analysis
Demographic data for the base cohort and the case source cohort were compared by Pearson’s Chi-square.
To calculate the risk of progression to TB, we adjusted for the sensitivity of the QFT-GIT for detecting the cases based on published data [12–18]. From each of these studies, we included the reported sensitivity and precision for the prediction of TB among QFT-GIT-positive individuals in a Bayesian model to obtain a posterior distribution of the sensitivity for the QFT-GIT, from which 20,000 random draws were taken to estimate disease progression and 95% credibility interval per 100,000 population [19]. The analyses were stratified for sex, age and TB incidence in the country of origin (online supplementary information). Associations were considered statistically significant when p-values were ≤0.05. All analyses were performed in SPSS 17.0 (Chicago, IL, USA) and WinBUGS 1.4.3 (Imperial College and MRC, London, UK).
RESULTS
Out of the 2,569 newly arrived immigrants, 1,570 (61%) gave informed consent, of whom 1,468 had a QFT-GIT result (fig. 1). Compared with nonconsenting subjects, the consenting subjects were significantly more often from Sub-Saharan Africa (p=0.001) and countries with high TB incidences (p<0.001). Reasons for individuals not to consent were predominantly a lack of time and fear of having blood drawn.
Figure 1–
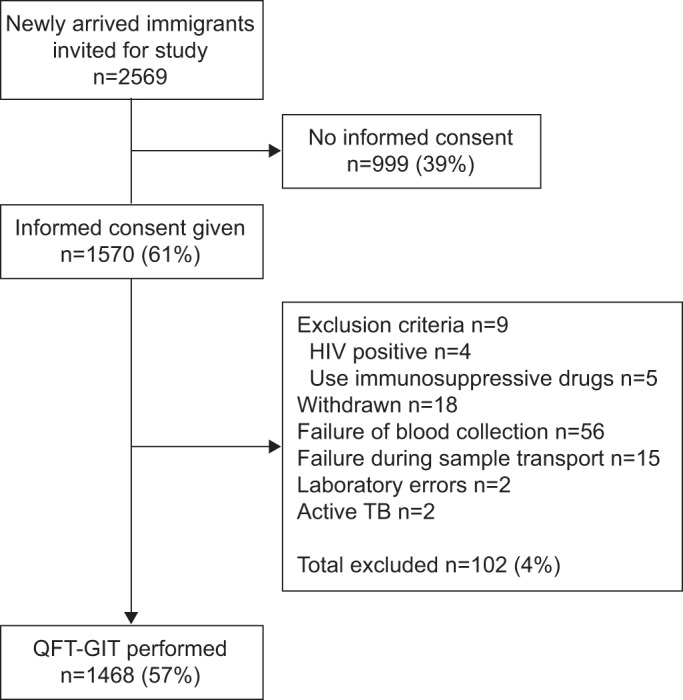
Study flow diagram of base cohort. TB: tuberculosis; QFT-GIT: QuantiFERON®-TB Gold In-Tube. (manufactured by Cellestis, Carnegie, Australia).
There were no significant differences between the base cohort and the case source cohort with regards to sex (p=0.086) or age (p=0.982), but moderate differences with regard to country of origin (p≤0.001) and incidence in the country of origin (p=0.006) (table 1).
Table 1– Baseline characteristics of the case source cohort and the base cohort including the number of QuantiFERON®-TB Gold In-Tube (QFT-GIT) positive subjects.
| Case source cohort# n (%) | Base cohort¶ | p-value | ||
| n (%) | QFT-GIT positive n (% of base cohort) | |||
| Total | 26317 (100) | 1468 (100) | 296 (20) | |
| Sex | ||||
| Female | 13766 (52) | 799 (54) | 152 (19) | 0.086 |
| Male | 12504 (48) | 669 (46) | 144 (22) | |
| Unknown | 47 (0) | 0 (0) | 0 (0) | |
| Age yrs | ||||
| 18–24 | 7877 (30) | 436 (30) | 58 (13) | 0.982 |
| 25–34 | 12797 (49) | 716 (49) | 163 (23) | |
| ≥35 | 5643 (21) | 316 (22) | 75 (24) | |
| Region of origin | ||||
| Europe and the Americas | 7647 (29) | 376 (26) | 48 (13) | <0.001 |
| Middle East and North Africa | 3680 (14) | 219 (15) | 54 (25) | |
| Other Asia | 10849 (41) | 679 (46) | 141 (21) | |
| Sub-Saharan Africa | 3894 (15) | 188 (13) | 52 (28) | |
| Other | 1 (0) | 6 (0) | 1 (17) | |
| Unknown | 246 (1) | 0 (0) | 0 (0) | |
| Estimated TB incidence in country of origin cases per 100000 population | ||||
| <100 | 13799 (52) | 725 (49) | 116 (16) | 0.006 |
| 100–199 | 7231 (28) | 453 (31) | 107 (24) | |
| ≥200 | 5040 (19) | 284 (19) | 72 (25) | |
| Unknown | 247 (1) | 6 (0) | 1 (17) | |
| Ever smoked daily | ||||
| No | NA | 1025 (70) | 207 (20) | |
| Yes | 433 (29) | 87 (20) | ||
| Unknown | 10 (1) | 2 (20) | ||
| Ever treated for TB | ||||
| No | NA | 1418 (97) | 280 (20) | |
| Yes | 17 (1) | 9 (53) | ||
| Unknown | 33 (2) | 7 (21) | ||
| Time between entry and screening months | ||||
| ≤3 | NA | 1353 (92) | 278 (21) | |
| ≥4 | 108 (7) | 17 (16) | ||
| Unknown | 7 (0) | 1 (14) | ||
| TB-related abnormalities on CXR | ||||
| No | NA | 1454 (99) | 288 (20) | |
| Yes | 14 (1) | 8 (57) | ||
QFT-GIT is manufactured by Cellestis (Carnegie, Australia). TB: tuberculosis; CXR: chest radiograph; NA: not available. #: three cohorts of immigrants who were registered in the Monitoring for Screening of Immigrants programme and were screened at arrival in 2005, 2006 or 2007; ¶: sample of newly arriving immigrants at seven Public Health Services between April 2009 and March 2011.
Among the base cohort, 296 participants tested QFT-GIT positive (20%) and five had indeterminate results (<1%). The percentage of QFT-GIT-positive subjects was lower in the lowest age category, but similar in the two highest age categories (table 1). Among immigrants from low incidence countries, the prevalence of QFT-GIT-positive subjects was lowest, while this was similar for immigrants coming from intermediate- or high-incidence countries (table 1).
Among the case source cohort, 30 cases were diagnosed with TB within 2 yrs after the entry screening (table 2). 25 (83%) cases were bacteriologically confirmed by culture, of which 10 cases were clustered (assessed via variable number tandem repeat) with another TB patient in the NTR. None of these were confirmed to be epidemiologically linked with a case in the Netherlands, suggesting that the cases acquired infection before having arrived into the Netherlands.
Table 2– Risk of progression to tuberculosis (TB) within 2 yrs for QuantiFERON®-TB Gold In-Tube (QFT-GIT)-positive and -negative subjects.
| Case source cohort n | TB within 2 yrs# n | Incidence of TB within 2 yrs per 100000 population | Expected QFT-GIT positivity at entry¶ n (%) | Estimated risk of progression to TB per 100000 population+ (95% credibility interval) | ||
| QFT-GIT positive | QFT-GIT negative | |||||
| Total | 26317 | 30 | 114 | 5306 (20) | 467 (314–603) | 25 (0–64) |
| Sex | ||||||
| Female | 13766 | 15 | 109 | 2619 (19) | 473 (318–611) | 24 (0–60) |
| Male | 12504 | 15 | 120 | 2691 (22) | 460 (310–595) | 27 (0–68) |
| Age yrs | ||||||
| 18–24 | 7877 | 6 | 76 | 1048 (13) | 473 (318–611) | 15 (0–39) |
| 25–34 | 12797 | 16 | 125 | 2913 (23) | 453 (305–586) | 28 (0–72) |
| ≥35 | 5643 | 8 | 142 | 1339 (24) | 493 (332–638) | 33 (0–83) |
| TB incidence in the country of origin cases per 100000 population | ||||||
| <100 | 13799 | 12 | 87 | 2208 (16) | 456 (307–589) | 18 (0–46) |
| 100–199 | 7231 | 12 | 166 | 1708 (24) | 590 (397–762) | 38 (0–97) |
| ≥200 | 5040 | 6 | 119 | 1278 (25) | 386 (259–499) | 28 (0–71) |
QFT-GIT is manufactured by Cellestis (Carnegie, Australia). #: based on surveillance data from Monitoring for Screening of Immigrants and the Netherlands Tuberculosis Register; ¶: number estimated based on QFT-GIT-positive prevalence in base cohort as presented in table 1; +: based on Bayesian statistics for posterior distribution resulted in a median (95% credibility interval) sensitivity for QFT-GIT of 83% (56–100%).
Assuming a similar prevalence of QFT-GIT-positive subjects in the case source cohort, 5,306 people in the case source cohort would be expected to be QFT-GIT positive, and the positive QFT-GITs would be distributed as shown in table 2. The median of the posterior distribution of the sensitivity of the QFT-GIT as assessed by Bayesian analyses was 83%, with a 95% credibilty interval of 56–100%.
The overall associated risk of disease progression for QFT-GIT-positive subjects was markedly higher compared with QFT-GIT-negative subjects, being 467 (95% credibilty interval 314–603) versus 25 (95% credibilty interval 0–64) per 100,000 population, respectively (table 2). The risk of progression to TB with a positive QFT-GIT did not differ between males and females, or between the different age groups. Irrespective of incidence in country of origin, the risk of progression to TB was markedly higher in QFT-GIT-positive subjects compared with QFT-GIT-negative subjects.
DISCUSSION
The present study shows that one-fifth of a representative sample of newly arriving immigrants in the Netherlands had LTBI as measured by QFT-GIT. The estimated risk of progression to TB within 2 yrs for QFT-GIT-positive subjects was substantial and significantly higher than the risk of progression among QFT-GIT-negative subjects, irrespective of immigrants' sex, age and TB incidence in the country of origin. The risk was significantly higher than the Dutch risk-group definition of an incidence of 50 per 100,000 population, based on a 10-fold higher incidence of TB compared with that in the autochthonous population. This indicates that, in this study, we have identified a new risk group, i.e. newly arriving immigrants with a positive QFT-GIT result. This finding suggests that using QFT-GIT might be of value in immigrant entry screening programmes.
The observed prevalence of QFT-GIT positivity of 20% among newly arriving immigrants is representative at a national level because there were no marked differences in demographic characteristics between the base cohort and the case source cohort. Other European countries reported a similar LTBI prevalence among newly arriving immigrants measured by interferon-γ release assay, but in general among asylum seekers, and only at regional level [18, 20].
The high risk of progression to TB among immigrants originating from intermediate incidence countries might be explained by a high prevalence of risk factors associated with progression to TB. We did not observe risk differences based on sex and age. Because we determined the risk of progression to TB of QFT-GIT-positive immigrants in a screening setting, as opposed to a setting with actual recent documented exposure, we found lower risk estimates compared with those of previous studies [10].
We excluded 22 cases who developed TB within 6 months after entry screening. Of these, only three were identified passively, which can be considered as a result of reactivation. The exclusion of such a small number of cases leads to a slight underestimation of the risk of progression to TB. Given that most cases diagnosed with TB within 6 months were detected actively (by entry screening), the role of QFT-GIT for the first 6 months would have been limited.
The usefulness of screening newly arriving immigrants for LTBI is continuously debated, and differs from the screening for active TB. While active TB can be a serious immediate threat to public health, LTBI in the short term is not. Wilson and Jungner [21] proposed several criteria to fulfil before a screening programme could be implemented. According to these criteria, prerequisites are the use of an accurate diagnostic test that can diagnose a health state that is curable according to a comprehensive and acceptable treatment regimen. Translating these criteria to this specific setting, screening newly arriving immigrants for infection should only be considered if the programme is well organised, resources are sufficient for initiating such a strategy and there is a willingness to treat. Up to now, the available diagnostic test (TST) was deemed insufficient, and the need for treatment was disputed, while programmatic limitations were envisioned [22].
We have shown that QFT-GIT seems to have the discriminatory ability to classify individuals for low and high risk of progression to active TB within 2 yrs. It is currently unknown whether the development of active TB beyond 2 yrs post-immigration is also associated with QFT-GIT results at the time of immigration. If such association is strong, then targeted treatment of all QFT-GIT-positive immigrants could have a significant impact on the overall number of active TB in immigrants. Molecular data from the NTR suggest that the majority of immigrants who progress to TB several years post-immigration are due to remote infections, which would increase the expected effectiveness of entry screening for LTBI [1].
To what extent QFT-GIT distinguishes recent from remote infection is unclear, but can have implications whether to start preventive therapy. Recently infected individuals are considered to be at greater risk for progressing to disease compared with individuals with a remote infection [23]. Among newly arriving immigrants, a high proportion of remote infections has to be expected, which cannot be distinguished from recent infections. Until new technologies are designed for distinguishing recent from remote infection, for example, by further developing tests for measuring promising latency antigens [24], we should consider all newly arriving immigrants with a positive QFT-GIT to have a substantial risk of progressing to TB.
A possible intervention in QFT-GIT positive newly arriving immigrants is to offer prophylactic treatment. The Dutch prophylactic treatment regimen was recently revised from 6 months of daily isoniazid (6H) to either 6H or 3 months of daily isoniazid plus rifampicin (3HR) for HIV-negative individuals who are suspected to have been recently infected and have a CXR without TB-related abnormalities. Preventive therapy is not considered among individuals who are suspected to have a remote infection. In a meta-analysis, it was shown that 3HR was comparable with standard therapy with 6H or 12H in terms of efficacy, the proportion of severe side-effects and mortality, while treatment adherence was either equal or greater among patients receiving 3HR [25]. Up to now, only a limited number of individuals have been treated with the new 3HR regimen in the Netherlands, but completion rates seem to be promising [1]. A meta-analyses showed that the median (95% confidence interval) hepatotoxicity rate, defined by elevated hepatic aminotransferases and/or symptoms of hepatitis, for individuals aged <35 yrs treated with 6–9H was 0.2% (0.1–0.3%) [26]. Offering preventive therapy to newly arriving immigrants who are QFT-GIT positive is therefore expected to be possible without major hepatotoxicity problems, especially as the majority is aged <35 yrs. However, the prevalence of chronic viral hepatitis is significant in certain regions and can, therefore, be expected to be higher among immigrants than among native Dutch individuals, with an associated increase in the risk of hepatotoxicity.
The efficacy of prescribing prophylactic treatment to newly arriving immigrants with a positive QFT-GIT result is directly related to the prevalence of multidrug-resistant (MDR)-TB in the countries of origin. Although a threat to the efficacy of preventive therapy, we believe this will not be a major issue in newly arriving immigrants, given that between 2006 and 2009 the prevalence of MDR-TB among all notified patients in the Netherlands was ∼1% [1]. However, when active TB develops in an immigrant who has received treatment for LTBI, the risk of MDR-TB should be suspected from the outset and empirical treatment should include that possibility.
The number needed to treat to prevent one case of TB within 2 yrs, given a positive QFT-GIT and an efficacy of 60% of prophylactic treatment, will be ∼350. The corresponding number needed to screen (NNS) will be ∼1,800. This NNS is comparable with that found for other mass screening programmes in the Netherlands.
An alternative for preventive therapy is to actively follow-up QFT-GIT-positive immigrants by periodical CXR screening. The coverage of the current voluntary 6-monthly follow-up screening for a period of 2 yrs offered to immigrants from high incidence countries is dropping as low as 34% [4]. Furthermore, half of the cases in our case source cohort were diagnosed with extrapulmonary TB and would not have been detected by CXR. Offering active follow-up to QFT-GIT-positive immigrants, even if mandatory, is therefore unlikely to be effective. Another alternative to treatment is to rely on a well-established contact-tracing strategy in the Netherlands. However, analyses of routine data have shown that, in first-generation immigrants, this strategy works suboptimally compared with native Dutch TB patients [27, 28].
The public health benefit of screening newly arriving immigrants for LTBI depends highly on the organisation of the programme. Menzies et al. [7] highlighted that, in screening programmes, <40% of the participants who could have benefited from preventive therapy actually did so. Reasons for this were no participation in the initial screening, not showing up for medical evaluation of positive tests, but also noncompliance of physicians in following treatment recommendations leading to noncompliance or refusal of therapy among individuals diagnosed with LTBI. There is some recent evidence that these negative indicators can be improved upon by using QFT-GIT compared with the conventional TST [29, 30]. In depth research is needed to gain insight into barriers and facilitators of a successful treatment programme. Based on the data collection at the seven PHSs, we are convinced that screening newly arriving immigrants for LTBI by QFT-GIT is achievable without major logistical difficulties.
Our work had several limitations. The estimates for risk of progression to TB were not derived in a prospective manner. We therefore had to assume that the cases were already infected at entry. Being infected in the Netherlands after entry was considered unlikely as none of the cases in our study were epidemiologically linked with other patients diagnosed in the Netherlands. The risk of progression to TB could have been attributed to travelling to the country of origin after the initial screening, but we had no data to incorporate this.
Data regarding re-migration within 2 yrs after the entry screening was lacking, and therefore the direction of potential bias was unknown. However, because the observed risk for QFT-GIT-positive immigrants to progress to active TB was around 10-fold higher than the level used for the Dutch risk group definition (50 cases per 100,000 population), it is unlikely that preferential mass re-migration of immigrants without active TB underlay the observed findings.
Another limitation is that the results cannot be extrapolated to newly arriving immigrant children (aged ≤18 yrs). Nationwide, this group accounts for approximately one-sixth of the total number of patients diagnosed with TB within 2 yrs after entry [1]. Compared with adults, the risk of progression to active TB in children is higher indicating that the use of QFT-GIT for detection and targeted treatment of LTBI in children might thus be even more effective [31].
Conclusions
The findings of this study among newly arriving immigrants to the Netherlands provide evidence that screening by QFT-GIT detects those at high risk of subsequent TB reactivation and that targeted interventions might lower the TB incidence among first generation immigrants. The next step will be to incorporate these findings in a cost-effectiveness analysis including several alternative entry screening strategies as well as alternative strategies for TB control, such as contact investigation. This will give evidence to reconsider the national immigrant screening programme.
Acknowledgments
The authors′ affiliations are as follows. C. Mulder: KNCV Tuberculosis Foundation, The Hague, and Center for Infection and Immunity Amsterdam (CINIMA), Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands; H. van Deutekom: Dept of Tuberculosis Control, Public Health Service, Amsterdam, the Netherlands; E.M. Huisman: Dept of Tuberculosis Control, Public Health Service, The Hague, the Netherlands; S. Toumanian: Dept of Tuberculosis Control, Public Health Service, Enschede, the Netherlands; B.F.P.J. Koster: Dept of Tuberculosis Control, Public Health Service, Utrecht, the Netherlands; W. Meijer-Veldman: Dept of Tuberculosis Control, Public Health Service, Brabant Zuidoost, Eindhoven, the Netherlands; J.H. van Loenhout-Rooyackers: Dept of Tuberculosis Control, Public Health Service, Region Nijmegen, Nijmegen, the Netherlands; M. Appel: Dept of Tuberculosis Control, Public Health Service, Groningen, the Netherlands; S.M. Arend: Dept of Infectious Diseases, Leiden University Medical Center, Leiden, the Netherlands; M.W. Borgdorff: Center for Infection and Immunity Amsterdam (CINIMA), Academic Medical Center, University of Amsterdam, Dept of Infectious Diseases, Public Health Service, and Dept of Clinical Epidemiology, Biostatistics and Bioinformatics, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands; F. van Leth: KNCV Tuberculosis Foundation, The Hague, Dept of Global Health, Academic Medical Center, University of Amsterdam, and Amsterdam Institute for Global Health and Development, Amsterdam, the Netherlands.We would like to acknowledge all participants, persons and institutions involved during the data collection. Besides all staff from the seven participating PHSs (GGD Amsterdam, Amsterdam; GGD Den Haag, the Hague; GGD Regio Twente, Enschede; GG&GD Utrecht, Utrecht; GGD Brabant Zuidoost, Eindhoven; GGD Regio Nijmegen, Nijmegen; Hulpverleningsdienst GGD Groningen, Groningen) these include R. Dubbink, W. de Haan, C. Runhaar Compper, M. Olsthoorn, K. Abdulkadir, S. Bairi, M-C. Engelen, A. Kougioumtzoglou and D. van Trier (all independent non-affiliated data collectors). QFT-GIT was performed by the laboratories: Streeklaboratorium Amsterdam, Amsterdam; Haga Ziekenhuis Den Haag, the Hague; Laboratorium Microbiologie Twente Achterhoek, Enschede; St. Antonius Ziekenhuis, Nieuwegein; PAMM, Veldhoven; UMC St. Radboud Nijmegen, Nijmegen; and the Laboratorium voor Infectieziekten Groningen, Groningen, all the Netherlands. We would like to thank J. van Rest, H. Schimmel and E. Slump (all at the KNCV Tuberculosis Foundation, The Hague) for preparing the data from the NTR and the MSI for the analyses.
Footnotes
This article has supplementary material available from www.erj.ersjournals.com
Support Statement
This work and its open access publication were supported by the Netherlands Organization for Health Research and Development (ZonMw, grant number 125010011).
Statement of Interest
None declared.
REFERENCES
- 1.KNCV Tuberculosis Foundation Tuberculose in Nederland 2009 [Tuberculosis in The Netherlands 2009]. KNCV Tuberculosis Foundation, The Hague, 2009 [Google Scholar]
- 2.van Leth F, Kalisvaart NA, Erkens CG, et al. Projection of the number of patients with tuberculosis in the Netherlands in 2030. Eur J Public Health 2009; 19: 424–427 [DOI] [PubMed] [Google Scholar]
- 3.Bwire R, Nagelkerke N, Keizer ST, et al. Tuberculosis screening among immigrants in The Netherlands: what is its contribution to public health? Neth J Med 2000; 56: 63–71 [DOI] [PubMed] [Google Scholar]
- 4.Erkens C, Slump E, Kamphorst M, et al. Coverage and yield of entry and follow-up screening for tuberculosis among new immigrants. Eur Respir J 2008; 32: 153–161 [DOI] [PubMed] [Google Scholar]
- 5.Vos AM, Meima A, Verver S, et al. High incidence of pulmonary tuberculosis persists a decade after immigration, The Netherlands. Emerg Infect Dis 2004; 10: 736–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricks PM, Cain KP, Oeltmann JE, et al. Estimating the burden of tuberculosis among foreign-born persons acquired prior to entering the U.S., 2005-2009. PLoS One 2011; 6: e27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzies D, Al Jahdali H, Al Otaibi B. Recent developments in treatment of latent tuberculosis infection. Indian J Med Res 2011; 133: 257–266 [PMC free article] [PubMed] [Google Scholar]
- 8.Borgdorff MW, van den Hof S, Kremer K, et al. Progress towards tuberculosis elimination: secular trend, immigration and transmission. Eur Respir J 2010; 36: 339–347 [DOI] [PubMed] [Google Scholar]
- 9.Pai M, Dheda K, Cunningham J, Scano F, et al. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis 2007; 7: 428–438 [DOI] [PubMed] [Google Scholar]
- 10.Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxlade O, Schwartzman K, Menzies D. Interferon-γ release assays and TB screening in high-income countries: a cost-effectiveness analysis. Int J Tuberc Lung Dis 2007; 11: 16–26 [PubMed] [Google Scholar]
- 12.Diel R, Loddenkemper R, Meywald-Walter K, et al. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med 2008; 177: 1164–1170 [DOI] [PubMed] [Google Scholar]
- 13.Kik SV, Franken WP, Mensen M, et al. Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. Eur Respir J 2010; 35: 1346–1353 [DOI] [PubMed] [Google Scholar]
- 14.Yoshiyama T, Harada N, Higuchi K, et al. Use of the QuantiFERON-TB Gold test for screening tuberculosis contacts and predicting active disease. Int J Tuberc Lung Dis 2010; 14: 819–827 [PubMed] [Google Scholar]
- 15.Diel R, Loddenkemper R, Niemann S, et al. Negative and positive predictive value of a whole-blood interferon-γ release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med 2011; 183: 88–95 [DOI] [PubMed] [Google Scholar]
- 16.Aichelburg MC, Rieger A, Breitenecker F, et al. Detection and prediction of active tuberculosis disease by a whole-blood interferon-γ release assay in HIV-1-infected individuals. Clin Infect Dis 2009; 48: 954–962 [DOI] [PubMed] [Google Scholar]
- 17.Mahomed H, Hawkridge T, Verver S, et al. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One 2011; 6: e17984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harstad I, Winje BA, Heldal E, et al. Predictive values of QuantiFERON-TB Gold testing in screening for tuberculosis disease in asylum seekers. Int J Tuberc Lung Dis 2010; 14: 1209–1211 [PubMed] [Google Scholar]
- 19.Ntzoufras I. Basyesian Modeling Using WinBUGS New York, Wiley, 2009 [Google Scholar]
- 20.Pareek M, Watson JP, Ormerod LP, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 2011; 11: 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva, World Health Organization, 1968 [Google Scholar]
- 22.Coker R, van Weezenbeek KL. Mandatory screening and treatment of immigrants for latent tuberculosis in the USA: just restraint? Lancet Infect Dis 2001; 1: 270–276 [DOI] [PubMed] [Google Scholar]
- 23.Borgdorff MW, Sebek M, Geskus RB, et al. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol 2011; 40: 964–970 [DOI] [PubMed] [Google Scholar]
- 24.Goletti D, Butera O, Vanini V, et al. Response to Rv2628 latency antigen associates with cured tuberculosis and remote infection. Eur Respir J 2010; 36: 135–142 [DOI] [PubMed] [Google Scholar]
- 25.Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005; 40: 670–676 [DOI] [PubMed] [Google Scholar]
- 26.Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis 2010; 14: 1374–1381 [PubMed] [Google Scholar]
- 27.Mulder C, Erkens CG, Kouw PM, et al. Missed opportunities in tuberculosis control in The Netherlands due to prioritization of contact investigations. Eur J Public Health 2012; 22: 177–182 [DOI] [PubMed] [Google Scholar]
- 28.Mulder C, van Deutekom H, Huisman EM, et al. Coverage and yield of tuberculosis contact investigations in the Netherlands. Int J Tuberc Lung Dis 2011; 15: 1630–1637 [DOI] [PubMed] [Google Scholar]
- 29.Grinsdale JA, Ho CS, Banouvong H, et al. Programmatic impact of using QuantiFERON®-TB Gold in routine contact investigation activities. Int J Tuberc Lung Dis 2011; 15: 1614–1620 [DOI] [PubMed] [Google Scholar]
- 30.van Leth F, Borgdorff M. Contact tracing in low-incidence tuberculosis settings. Int J Tuberc Lung Dis 2011; 15: 1566. [DOI] [PubMed] [Google Scholar]
- 31.Bakir M, Millington KA, Soysal A, et al. Prognostic value of a T-cell-based, interferon-γ biomarker in children with tuberculosis contact. Ann Intern Med 2008; 149: 777–787 [DOI] [PMC free article] [PubMed] [Google Scholar]


