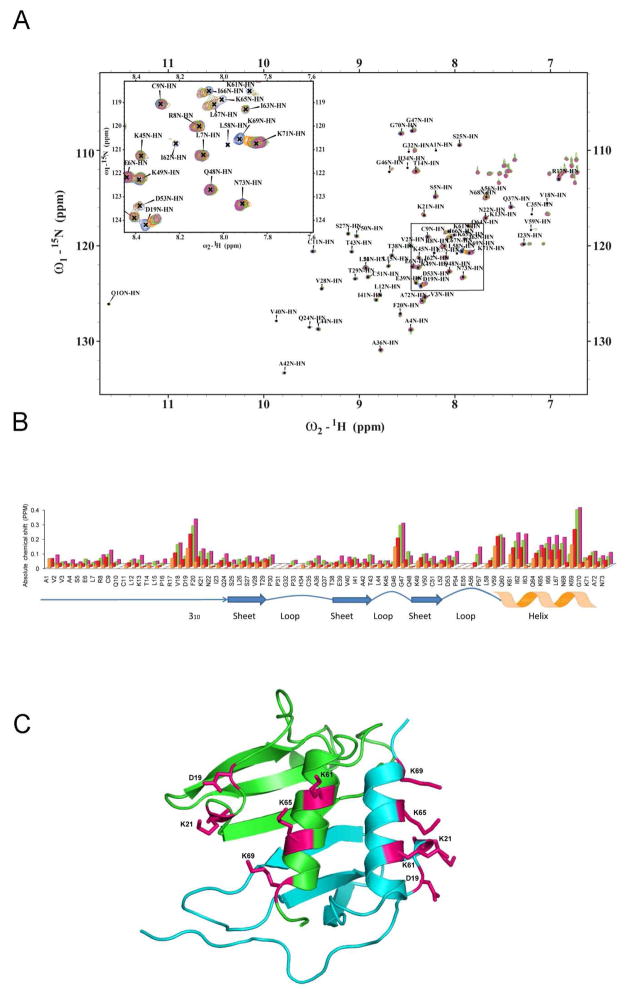
Figure 3.
NMR spectroscopic studies of murine MIP-2 and heparin disaccharide interaction. A, NMR chemical shift changes induced by titration of heparin disaccharide I-S. 1H-15N-HSQC spectra of 300μM 15N-MIP-2 (blue) are overlaid with spectra from molar ratios of 15N-MIP-2 to disaccharide of 1:2, 1:4, 1:8, and 1:16 (orange, red, green, and pink), respectively. Peak labels indicate peaks for the uncomplexed (apo) protein. B, Absolute NMR chemical shift change of each residue for the disaccharide titration. Absolute NMR chemical shift change for each ratio are calculated as (((|Nppm-bound − Nppm-apo|) + (|Hppm-bound − Hppm-apo| × 6.5))/2). Uncomplexed 15N-MIP-2 compared to 15N-MIP-2:heparin disaccharide I-S ratios of 1:2 (orange), 1:4 (red), 1:8 (green), and 1:16 (pink). Changes in NMR chemical shifts for proline residues are reported as zero as they lack an amide proton. Regions of secondary structure are indicated below with block arrows representing β regions and zigzags representing helical regions. C, The chemical shifts are mapped and the residues are shown as sticks on a ribbon diagram of the crystallographic dimeric (green and blue monomers) structure of MIP-2.