Abstract
Force induced transformations of polymer bound functionalities have the potential to produce a rich array of stress responsive behavior. One area of particular interest is the activation of non-scissile mechanophores in which latent reactivity can be unveiled that, under the appropriate conditions, could lead to constructive bond formation in materials exposed to typically destructive stress. Here, the mechanical activation of a bicyclo[3.2.0]heptane (BCH) mechanophore is demonstrated via selective labeling of bis-enone products. BCH ring-opening produces large local elongation (> 4 Å) and products that are reactive to conjugate additions under mild conditions. Subsequent photocyclization regenerates the initial BCH functionality, providing switchable structure and reactivity along the polymer backbone in response to stress and visible light.
Mechanochemically active polymers have the potential to create a new generation of stress responsive and self-healing materials. 1,2 It has been proposed that stress-induced degradation of polymer chains on the molecular level, a mechanism that contributes to failure in bulk materials, could potentially be attenuated by incorporating latent reactive groups that can elicit constructive bond forming reactions in regions of high stress. Additionally, non-scissile mechanochemical ring-opening events may create “on demand” regions of local slack under applied mechanical load, thus preventing catastrophic failure.3,4 Many examples of stress-responsive mechanophores have been reported,5–15 and they have proved invaluable in studying the rich array of reactivity and stereochemical outcomes available through mechanochemistry. Despite this progress, most mechanophores to date, when activated, result in scission of their parent polymer chain. Examples of non-scissile mechanophores include: benzocyclobutenes (BCBs),5,6 binols, 7 epoxides,16 cyclobutene molecular force probes,17 and gem-dihalocyclopropanes (gDHCs).13 These generally offer only modest (1–2 Å) and sometimes transient extensions, and only select gDHCs and cyclobutenes generate products with both good stability and significant elongations.
We therefore set out to construct a non-scissile mechanophore with substantial stored length and a stable activation product that is cross-reactive with uncharged reagents. The extensive palette of existing scissile mechanophores provided a rich set of reactivities from which to draw inspiration. In particular, we took note of the recent report by Moore,18 in which cyclobutanes were mechanically cleaved to enones. We hypothesized that the mechanophores could be converted from scissile to non-scissile by incorporating them into a fused, bicyclic framework such as a bicyclo[3.2.0]heptane (BCH). In addition to non-scissile behavior and good product reactivity, we envisaged that this design would give large extensions compared to the aforementioned examples, as well as allow for new, two-way switching behavior via photochemical reversion of the bis-enone products to the starting BCH mechanophore. Here, we report the synthesis and mechanochemical activation of a BCH mechanophore. Features of this system include nonscissile activation, generation of reactive enones, large local elongation, and photochemical reversibility.
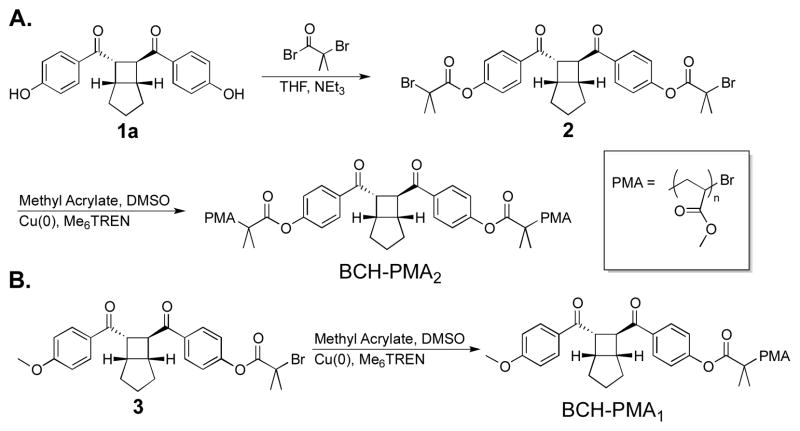
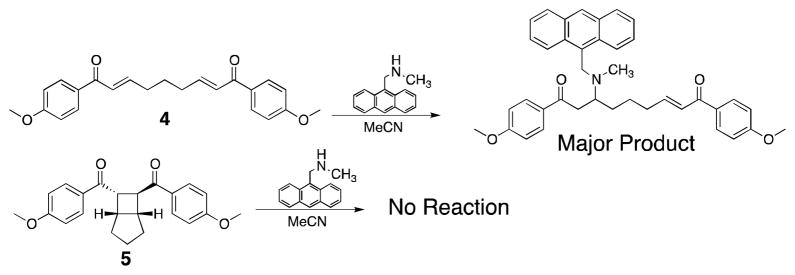
To access the BCH architecture, we looked to the simple and efficient synthesis of such molecules by Yoon.19 Bisphenol 1a was reacted with alpha-bromoisobutyryl bromide to yield 2, a BCH-centered difunctional initiator (Scheme 1a). Reaction of 2 under standard SET-LRP conditions20,21 resulted in chain-centered BCH-PMA2,N with various molecular weights (N = MW). Additionally, a similar strategy was used to access monofunctional initiator 3, for the synthesis of end-functional control polymer BCH-PMA1 (Scheme 1b). Chain-end linked mechanophores are commonly used as control systems in pulsed ultrasound as shear stresses accumulate towards the center of the chain, precluding activation at the chain end. The low mechanophore content in these systems generally precludes direct observation of the reaction outcome by 1H NMR, and the most common strategy to detect activation is to label the reaction products with UV active functionalities.5,8,10,22 To ensure orthogonal labeling of the proposed bis-enone product over the starting BCH functionality, we explored the use of conjugate addition by secondary amines (Scheme 2).23 When small molecule analogue 522 was exposed to 9-methylaminomethyl anthracene (MAMA) (5 equiv, 0.25 M) in MeCN overnight, no change was observed in the 1H NMR spectrum. Alternatively, when a bis-enone analogue 4 was subjected to identical conditions, the primary product observed was the mono-MAMA adduct (see Supporting Information). These observations motivated our experimental design, whereby activation of the BCH functionality by pulsed ultrasound could be tracked by the addition of MAMA to bis-enone products.
Scheme 1.
Synthesis of BCH-linked PMA via SET-LRP of diinitiator 3, and end-functional control BCH-PMA1.
Scheme 2.
Orthogonal labeling demonstrated by small molecule reactivity.
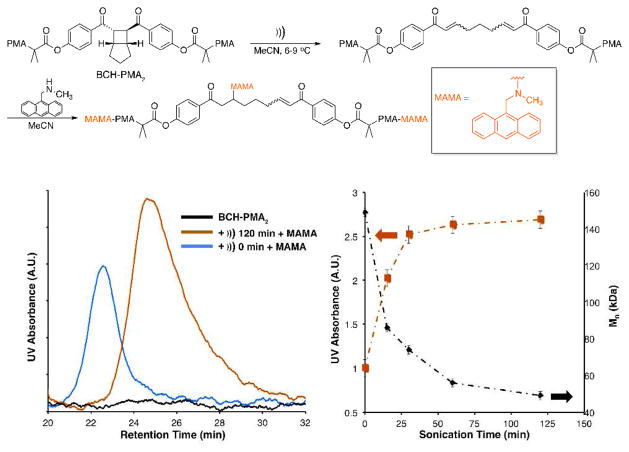
When BCH-PMA2,151k is subjected to pulsed ultrasound in acetonitrile (6–9 °C) and subsequently treated with 0.09 M MAMA, the integral of the UV absorbance (365 nm) increases with increasing sonication time (Figure 1). After 120 minutes this increase levels off at a ratio of 2.7:1 vs. the unsonicated sample (0 min) or a value of χMAMA = 0.81 (Table 1), which we define as additional molecules of MAMA incorporated per initial chain relative to an unsonicated control (see Supporting Information). Because the MAMA concentration used with the polymers is lower than that used with small molecule analogue 4, we expect that the labeling is dominated by formation of the mono-MAMA adduct.
Figure 1.
Sonochemical activation and subsequent labeling of bis-enone with MAMA (top). GPC-UV traces (365 nm, left) shows no absorbance in the native polymer (BCHPMA2) (black), the absorbance of the unsonicated polymer treated with MAMA (blue), and the absorbance of the sonicated (120 min) polymer treated with MAMA (orange). Increasing UV absorbance and concurrent MW degradation is plotted at various time points during sonication (right).
Table 1.
Summary of PMA Sonication and Labeling Experiments.
| Polymer | Mn | PDI | Mn,))) | Absinit | Abs))) | χMAMA |
|---|---|---|---|---|---|---|
| BCH-PMA2,151k | 151 | 1.11 | 49 | 0.039 | 0.119 | 0.81 |
| PMACRP,149k | 149 | 1.11 | 54 | 0.073 | 0.088 | 0.15 |
| PMAFRP,157k | 157 | 2.12 | 51 | N/A | 0.006 | 0.12 |
| BCH-PMA2,23k | 23 | 1.08 | 23 | 0.210 | 0.279 | 0.11 |
| BCH-PMA1,158k | 158 | 1.12 | 61 | 0.021 | 0.030 | 0.09 |
Polymers of molecular weight Mn were subjected to pulsed ultrasound in acetonitrile (6–9 °C) for 120 min and subsequently treated with MAMA (0.09 M) overnight. Absorbance (365 nm) is given for the polymer treated with MAMA with (Abs)))) and without (Absinit) sonication.
We note the presence of UV absorbance in the unsonicated control polymer after treatment with MAMA (Table 1, BCHPMA2,151k). This can be attributed to addition of the secondary amine to the chain end α-bromoesters that result from the SET-LRP process. Due both to terminal group reactivity and to the non-scissile nature of the mechanophore, the change in absorbance does not directly correlate with molecular weight degradation, as mechanophore activation can occur independently from chain scission. For this reason, the absorbance is seen to increase in both the high (unbroken chains) and low (broken chains) molecular weight regions of the GPC trace (Figures S7–S9 in Supporting Information).
The terminal bromide is highly susceptible to nucleophilic attack, as demonstrated by the “click” reaction with thiophenols described by the Percec group.24 In support of this mechanism, PMA homopolymer (PMACRP,149k) produced by the same method shows a similar increase in absorbance when reacted with MAMA, with minimal additional increase after sonication (χMAMA = 0.15). We note that the modest increase in UV absorbance in the control sample is not unusual, and has been observed after sonication in similar labeling experiments. 22 Alternatively, PMA prepared by conventional free radical polymerization (PMAFRP,157k) shows no UV absorbance due to lack of halogenated endgroups. Low molecular weight BCH-PMA2,23k also exhibits an increase in absorbance upon treatment with MAMA, and, upon purification by preparatory GPC, its 1H NMR spectrum shows no change in the BCH functionality. Upon sonication, this polymer exhibits only a mild increase in UV Absorbance (χMAMA = 0.11). Similar behavior is observed when control polymer BCH-PMA1 bearing chain-end BCH functionality is subjected to identical conditions, yielding a χMAMA value of 0.09, both results indicating that mechanochemical, rather than thermal processes, drive this ring-opening reaction.
These results demonstrate that the mechanochemical activity of the fused BCH is qualitatively similar to that of Moore’s cyclobutanes that inspired the design. Polymer16 and mechanophore 18,25 architecture can have significant and sometimes unexpected effects on mechanical activity, however, and we turned to CoGEF calculations26 to quantify the impact of the BCH framework on mechanical activity of the cyclobutane. In the CoGEF calculations, the BCH functionality is seen to transform into the corresponding bis-enone product via a formal retro [2+2] cycloaddition at 4.08 Å elongation. Both this extension at break and the associated force (6.01 nN) are in excellent agreement with that of similar trans substituted cyclobutane mechanophores previously reported by the Moore group.18
The second ring system appears to fulfill its desired role as an otherwise inert source of stored length. That stored length has the potential to generate large amounts of local slack and increased strain windows between activation and rupture in polymer chains or sub-chains,4 concepts that motivated our design. Quantitatively, we are able to estimate the elongation upon activation using our CoGEF force-displacement relationship. The contour lengths before and after activation were calculated at 100 pN of simulated force (estimating the force necessary to exhaust conformational entropy of a single polymer chain), showing a local elongation of 4.31 Å, which to our knowledge is the largest extension of any non-scissile mechanophore to date (see Supporting Information).
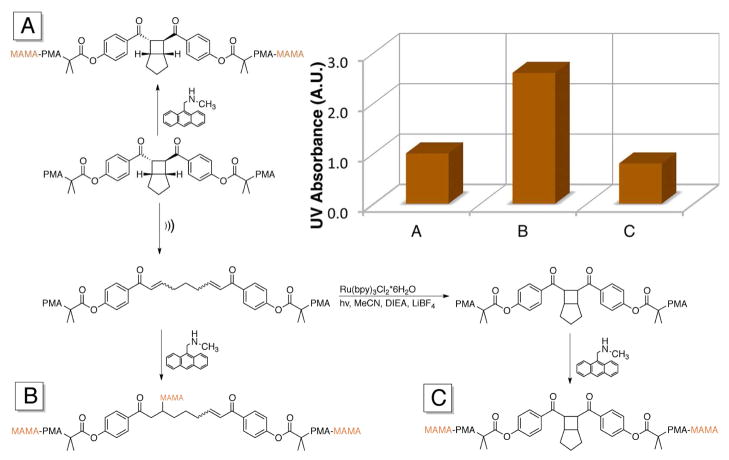
That extension in contour length can only be realized, however, if the mechanophore is truly non-scissile. That is, the stored length that is released by the retro-cycloaddition must survive the forces required for ring opening. That this is the case is demonstrated by recyclizing the bis-enone under the same Ru(bpy)3Cl2 sensitized photocyclization used to generate the starting BCH small molecule 1a (Product C, Fig. 2).19 Sonication and reaction with MAMA (Product B) generates a ~2.6 fold increase in absorbance over the control (Product A), whereas the final UV absorbance from Product C drops appreciably to ~0.8 vs. the control. The photoreversion occurs without a change in MW, confirming an intramolecular, rather than intermolecular, addition of the enones. The chain scission process is therefore spatially and temporally distinct from the BCH activation. Reversible mechanochemistry has been demonstrated for several scissile mechanophores,8,10,15,27 but reversibility in non-scissile mechanophores has been limited to the mechanical/thermal cis/trans isomerization of gem-difluorocyclopropanes, 14,28 switching that comes with no significant difference in intrinsic reactivity between the two states. We believe the BCH mechanophore to be the first example of reversible, non-scissile mechanophores with states of qualitatively different chemical reactivity, and the first example of photoreversion of an activated mechanophore of any type.
Figure 2.
Summary of activation and recyclization experiment. A: Initial polymer treated with MAMA. B: After sonication and treatment with MAMA. C: After recyclization and treatment with MAMA. Absorbance intensity returns to presonication level after photochemical recyclization (inset).
In conclusion, we have demonstrated the mechanochemical generation of bis-enones via the formal retro [2+2] cycloaddition of a non-scissile bicyclo[3.2.0]heptane based mechanophore. The functionality generated upon activation can participate in conjugate additions that we envision could allow for application in stress-responsive and/or self-healing materials. Furthermore, this reaction is reversible under photochemical conditions, demonstrating a platform where damage due to stress induced ring opening potentially could be reversed by ambient sunlight. Currently we are pursuing the synthesis of high mechanophore content analogues29 of this system for applications along these lines.
Supplementary Material
Acknowledgments
This material is based on work supported by the U.S. Army Research Laboratory and the Army Research Office under Grant W911NF-07-1-0409. ZSK thanks the NIH for a NIGMS Biotechnology Predoctoral Training Grant (T32GM8555). SLC acknowledges support from the NSF (DMR-1122483). We thank Jeff Moore and Matt Kryger for encouraging conversations.
Footnotes
Supporting Information. Synthetic details, NMR, GPC, UV characterization, control experiment details, CoGEF calculations, and additional details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Caruso MM, Davis DA, Shen Q, Odom SA, Sottos NR, White SR, Moore JS. Chem Rev. 2009;109:5755–5798. doi: 10.1021/cr9001353. [DOI] [PubMed] [Google Scholar]
- 2.Black AL, Lenhardt JM, Craig SL. J Mater Chem. 2011;21:1655–1663. [Google Scholar]
- 3.Kean ZS, Craig SL. Polymer. 2012;53:1035–1048. [Google Scholar]
- 4.Wu D, Lenhardt JM, Black AL, Akhremitchev BB, Craig SL. J Am Chem Soc. 2010;132:15936–15938. doi: 10.1021/ja108429h. [DOI] [PubMed] [Google Scholar]
- 5.Hickenboth CR, Moore JS, White SR, Sottos NR, Baudry J, Wilson SR. Nature. 2007;446:423–427. doi: 10.1038/nature05681. [DOI] [PubMed] [Google Scholar]
- 6.Potisek SL, Davis DA, Sottos NR, White SR, Moore JS. J Am Chem Soc. 2007;129:13808–13809. doi: 10.1021/ja076189x. [DOI] [PubMed] [Google Scholar]
- 7.Wiggins KM, Hudnall TW, Shen QL, Kryger MJ, Moore JS, Bielawski CW. J Am Chem Soc. 2010;132:3256–3257. doi: 10.1021/ja910716s. [DOI] [PubMed] [Google Scholar]
- 8.Brantley JN, Wiggins KM, Bielawski CW. Science. 2011;333:1606–1609. doi: 10.1126/science.1207934. [DOI] [PubMed] [Google Scholar]
- 9.Wiggins KM, Hudnall TW, Tennyson AG, Bielawski CW. J Mater Chem. 2011;21:8355–8359. [Google Scholar]
- 10.Wiggins KM, Syrett JA, Haddleton DM, Bielawski CW. J Am Chem Soc. 2011;133:7180–7189. doi: 10.1021/ja201135y. [DOI] [PubMed] [Google Scholar]
- 11.Karthikeyan S, Potisek SL, Piermattei A, Sijbesma RP. J Am Chem Soc. 2008;130:14968–14969. doi: 10.1021/ja806887k. [DOI] [PubMed] [Google Scholar]
- 12.Paulusse JMJ, Huijbers JPJ, Sijbesma RP. Chemistry – A European Journal. 2006;12:4928–4934. doi: 10.1002/chem.200600120. [DOI] [PubMed] [Google Scholar]
- 13.Lenhardt JM, Black AL, Craig SL. J Am Chem Soc. 2009;131:10818–10819. doi: 10.1021/ja9036548. [DOI] [PubMed] [Google Scholar]
- 14.Lenhardt JM, Ong MT, Choe R, Evenhuis CR, Martinez TJ, Craig SL. Science. 2010;329:1057–1060. doi: 10.1126/science.1193412. [DOI] [PubMed] [Google Scholar]
- 15.Klukovich HM, Kean ZS, Iacono ST, Craig SL. J Am Chem Soc. 2011;133:17882–17888. doi: 10.1021/ja2074517. [DOI] [PubMed] [Google Scholar]
- 16.Klukovich HM, Kean ZS, Ramirez ALB, Lenhardt JM, Lin J, Hu X, Craig SL. J Am Chem Soc. 2012;134:9577–9580. doi: 10.1021/ja302996n. [DOI] [PubMed] [Google Scholar]
- 17.Yang QZ, Huang Z, Kucharski TJ, Khvostichenko D, Chen J, Boulatov R. Nature Nanotechnology. 2009;4:302–306. doi: 10.1038/nnano.2009.55. [DOI] [PubMed] [Google Scholar]
- 18.Kryger MJ, Munaretto AM, Moore JS. J Am Chem Soc. 2011;133:18992–18998. doi: 10.1021/ja2086728. [DOI] [PubMed] [Google Scholar]
- 19.Ischay MA, Anzovino ME, Du J, Yoon TP. J Am Chem Soc. 2008;130:12886–12887. doi: 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]
- 20.Lligadas G, Rosen BM, Monteiro MJ, Percec V. Macromolecules. 2008;41:8360–8364. [Google Scholar]
- 21.Berkowski KL, Potisek SL, Hickenboth CR, Moore JS. Macromolecules. 2005;38:8975–8978. [Google Scholar]
- 22.Kryger MJ, Ong MT, Odom SA, Sottos NR, White SR, Martinez TJ, Moore JS. J Am Chem Soc. 2010;132:4558–4559. doi: 10.1021/ja1008932. [DOI] [PubMed] [Google Scholar]
- 23.Jung ME. In: Comprehensive Organic Synthesis. Barry MT, Ian F, editors. Pergamon; Oxford: 1991. pp. 1–67. [Google Scholar]
- 24.Rosen BM, Lligadas G, Hahn C, Percec V. J Polym Sci, Part A: Polym Chem. 2009;47:3940–3948. [Google Scholar]
- 25.Brantley JN, Konda SSM, Makarov DE, Bielawski CW. J Am Chem Soc. 2012;134:9882–9885. doi: 10.1021/ja303147a. [DOI] [PubMed] [Google Scholar]
- 26.Beyer MK. J Chem Phys. 2000;112:7307–7312. [Google Scholar]
- 27.Chen X, Wudl F, Mal AK, Shen H, Nutt SR. Macromolecules. 2003;36:1802–1807. [Google Scholar]
- 28.Lenhardt JM, Ogle JW, Ong MT, Choe R, Martinez TJ, Craig SL. J Am Chem Soc. 2011;133:3222–3225. doi: 10.1021/ja107645c. [DOI] [PubMed] [Google Scholar]
- 29.Kean ZS, Ramirez ALB, Craig SL. J Polym Sci, Part A: Polym Chem. 2012 doi: 10.1002/pola.26148. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.