Figure 5.
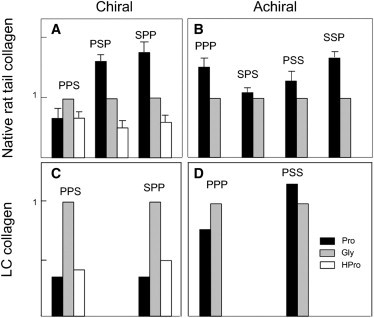
Relative amounts of amide I subbands for achiral and chiral spectra in RTT and LC collagen. Peak areas corresponding to the three major amide I subbands present in collagen were computed based on curve-fitting data (Fig. 4). Based on analogy to IR spectra, the band assignments correspond to the collagen tripeptide residues: Gly (mid-frequency, gray bar), Pro (low-frequency, black bar), Hpro (high-frequency, white bar). Error bars in 5 A and 5 B represent the standard deviation (determined from three RTT samples). The peak area of Gly was arbitrarily set to 1, and the areas for the other subbands were adjusted accordingly. Data for chiral and achiral spectra for both native RTT and LC collagen are shown in the four panels: (A) chiral spectra, native RTT; (B) achiral spectra, native RTT; (C) chiral spectra, LC collagen; (D) achiral spectra, LC collagen. Note the absence of Hpro in all achiral spectra.
