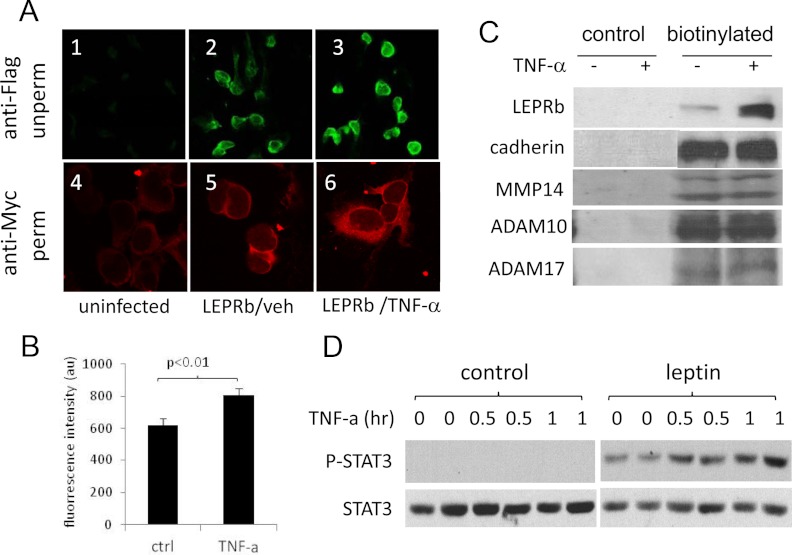
Fig. 4.
TNF-α up-regulates cell surface expression of LEPRb and cellular response to leptin. A, Confocal images of HEK293 cells stained for LEPRb. Ad-LEPRb-infected cells were treated for 6 h with vehicle (LEPRb/veh, panels 2 and 5) or with 100 ng/ml of TNF-α (LEPRb/TNF-α, panels 3 and 6). Cell surface LEPRb were stained by indirect immunofluorescence with anti-FLAG antibody in the unpermeabilized (unperm) condition (panels 1–3; ×20 objective), or permeabilized (perm) and stained by indirect immunofluorescence with anti-Myc antibody (panels 4–6; ×100 objective). Uninfected cells (panels 1 and 4) were included as controls for nonspecific staining. B, FACS analysis of cell surface LEPRb levels. Ad-LEPRb-infected HEK293 cells were treated for 2 h with vehicle control (ctrl) or 50 ng/ml TNF-α. After the treatment, cell surface LEPRb was labeled by indirect immunofluorescence staining with anti-FLAG antibody in the unpermeabilized condition. Fluorescence intensity was quantified by FACS. C, Western blot analysis of cell surface expression of LEPRb and the endogenous cadherin, MMP14, ADAM10, and ADAM17 genes. Ad-LEPRb-infected HEK293 cells were treated for 20 h with vehicle (−) or 20 ng/ml TNF-α (+). Cell surface proteins were biotinylated with EZ-Link Sulfo-NHS-LC-Biotin and captured with NeutrAvidin beads and analyzed by Western blot using anti-Myc antibody. Controls were treated and analyzed the same in parallel except that they were not biotinylated. D, Western blot of phospho-STAT3 (P-STAT3) and STAT3. Quiescent LEPRb-transfected HEK293 cells were treated with 1 ng/ml TNF-α for 0, 0.5, and 1 h as indicated. Without removing TNF-α, cells were then stimulated with or without 50 ng/ml leptin for 15 min. Cell lysates were analyzed for phospho-STAT3 and STAT3 by Western blotting.