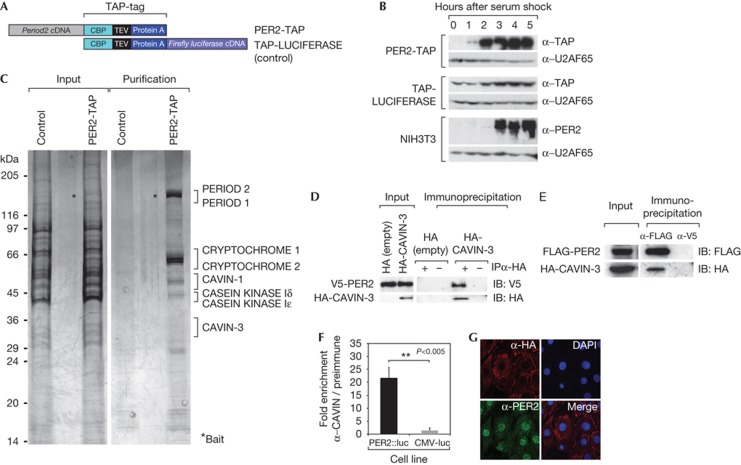
Figure 1.
CAVIN-3 is a new PER2 interaction partner. (A) Schematic representation of TAP-tagged proteins used for the purification. (B) Immunoblot showing the serum induction of PER2-TAP and TAP-LUCIFERASE in the stable cell lines, and endogenous PER2 in NIH3T3 cells. U2AF65 served as a loading control. (C) Silver stain of purified protein complexes separated by 8–16% SDS–polyacrylamide gel electrophoresis. (D) Co-immunprecipitation of V5-tagged PER2 with HA-CAVIN-3. The pCI-HA empty vector was used as a negative control. A negative control IP was performed with beads devoid of antibodies. (E) Co-immunoprecipitation of HA-CAVIN-3 with FLAG-PER2. V5 antibody was used as an irrelevant mouse monoclonal antibody (negative control). (F) CAVIN-3 was immunoprecipitated from Per2::Luc cells with anti-CAVIN-3 serum. Co-immunoprecipitated PER2::Luciferase was assessed as luciferase activity. The fold enrichment represents the luciferase activity normalized to the signal from IPs with pre-immune serum. CMV-luc stable cells served as a control. (G) HA-CAVIN-3 localizes to the cytoplasm. HA-CAVIN-3-expressing synchronized NIH3T3 cells were stained with HA and PER2 antibodies. Nuclei were stained with DAPI. Merge: DAPI and α-HA straining. DAPI, 4,6-diamidino-2-phenylindole; HA, haemagglutinin; IB, immunoblotting; IP, immunoprecipitation; PER, Period.