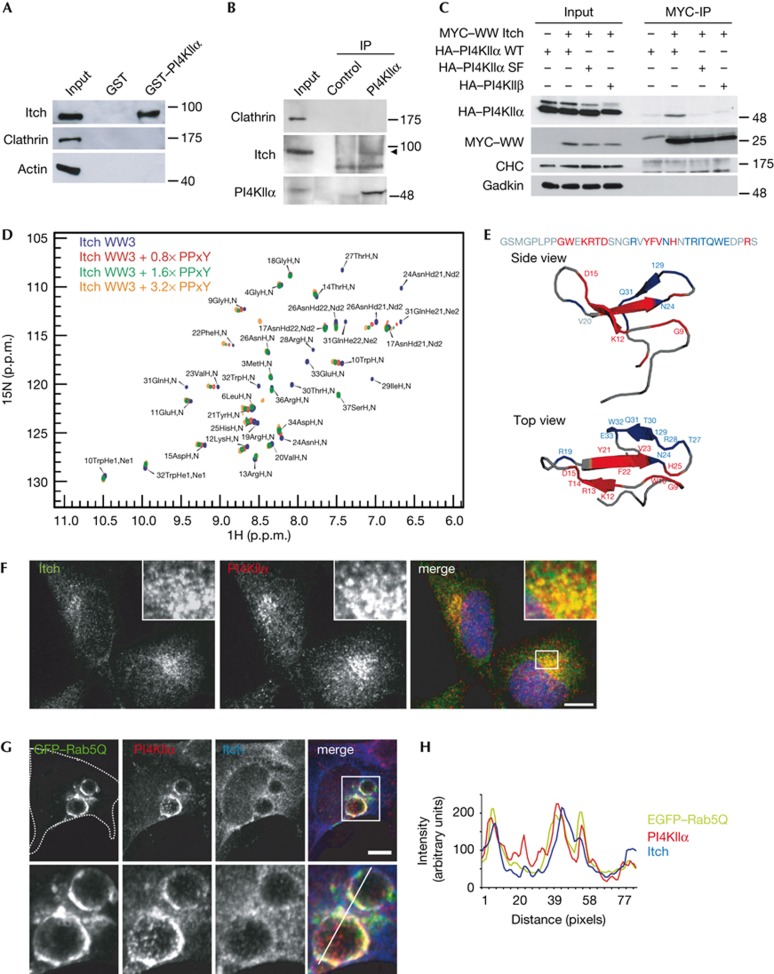
Figure 2.
PI4KIIα directly associates with the WW domains of the E3 ligase Itch. (A) GST–PI4KIIα co-purifies Itch, but not clathrin or actin from rat brain extracts. (B) Endogenous co-immunoprecipitation of PI4KIIα and Itch from uterus membrane extract. Arrow indicates Itch. (C) HEK293 cells stably expressing HA–PI4KIIα WT, HA–PI4KIIα SF or HA–PI4KIIβ were transfected with MYC-Itch WW domains (WW1-4). PI4KIIα WT specifically co-immunoprecipitated with Itch WW domain. Molecular weight markers in kD. (D) Overlay of the 1H, 15N HSQC spectra of 15N-WW3 Itch alone with spectra from samples containing increasing amounts of PI4KIIα PPxY peptide (no peptide, 0.8 × , 1.6 × and 3.2 × molar equivalents). (E) Sequence and cartoon models of Itch-WW3 (PDB ID: 2JO9). Red, NH resonances of residues that show chemical shift changes above average in D. Blue, residues disappearing on ligand addition. (F) Confocal images of endogenous PI4KIIα and Itch in HeLa cells. Pearson: PI4KIIα/Itch: 0.75. Scale bar, 12 μm. (G) Confocal images of endogenous PI4KIIα and Itch in HEK cells overexpressing eGFP–Rab5(Q79L). Cell borders indicated by dotted line. Lower panel, magnification of boxed area. Scale bar, 6 μm. (H) Fluorescence intensities along line shown in G plotted over distance. GST, glutathione S-transferase; HA, haemagglutinin; IP, immunoprecipitation; PI4KIIα, phosphatidylinositol 4-kinase type IIα; WT, wild-type.