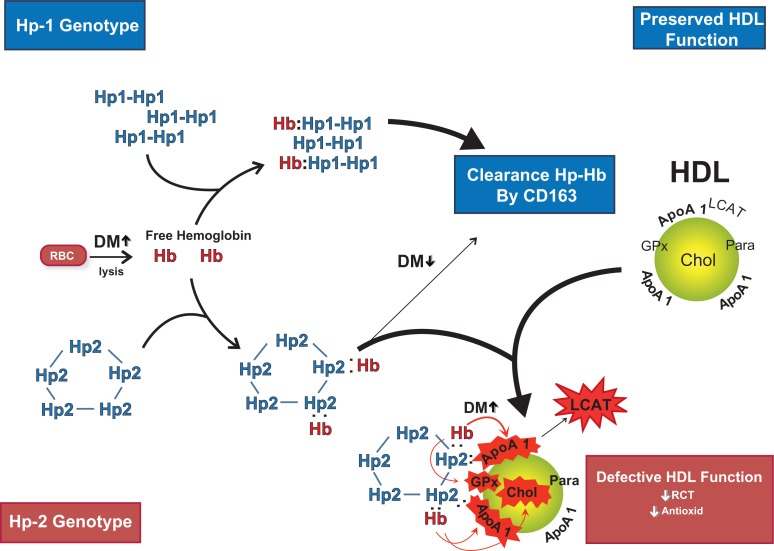
Figure 1.
Hemoglobin (Hb) released intravascularly from red blood cells (RBC) is rapidly bound by haptoglobin (Hp) protein to form an Hp–Hb complex. In Hp 2 diabetes mellitus (DM) individuals the complex is cleared more slowly than in Hp 1 DM individuals by the scavenger receptor CD163. The Hp–Hb complex can bind to Apo A1 in high-density lipoprotein (HDL), with increased binding of Hp 2–Hb occurring due its increased avidity for HDL and its increased plasma concentration. The Hp 2–Hb, but not the Hp 1–Hb complex, when bound to HDL can produce reactive oxygen species which can oxidize protein (ie, ApoA1; GPx-glutathione peroxidase; LCAT) and lipid components (cholesterol) of HDL and render the HDL dysfunctional (due to decreased reversed cholesterol transport [RCT] and antioxidant activity) proatherogenic and prothrombotic. Copyright © 2008. Reproduced with permission from Asleh R, Blum S, Kalet-Litman S, et al Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2–2 genotype. Diabetes. 2008; 57:2794–2800.