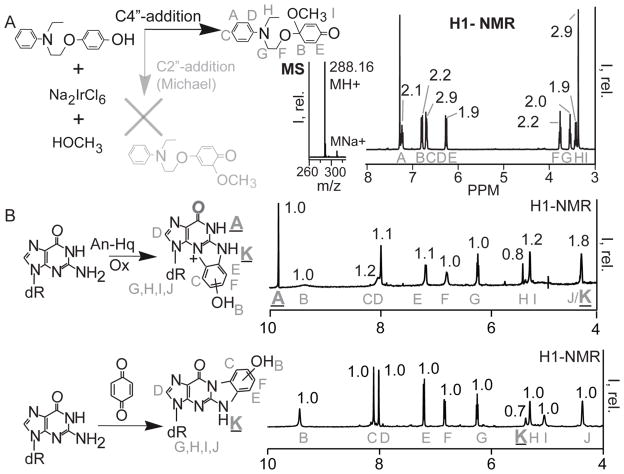
Figure 2. An-Hq and its Derivatives React with Guanine Differently than Benzoquinone.
(A) Quinones can react via two paths: C4″ addition, black, and C2″-Michael addition, grey. The product for each path is shown. To determine the reaction path, the product from oxidation in methanol was isolated. and the NMR and MS are shown. Oxidative activation of An-Hq in methanol proceeds via C4″ addition based on the grey NMR assignments and integrals. (B) Top. NMR of the reaction between An-Hq, 2′-deoxyguanosine, and an iridium oxidant in phosphate buffer. On the left is the reaction with the assigned NMR resonances superimposed on the product. On the right is the H1-NMR between 4–10 ppm. The assignments are in grey. An N1-proton signal is observed. Bottom. The reaction between 2′-deoxyguanosine, base, and benzoquinone according to literature. On the left is the reaction with assignments. On the right is the H1-NMR. Importantly, the N1 peak at around 10ppm is missing from the benzoquinone reaction product NMR. The different reaction path of An-Hq leads to a different guanine lesion.