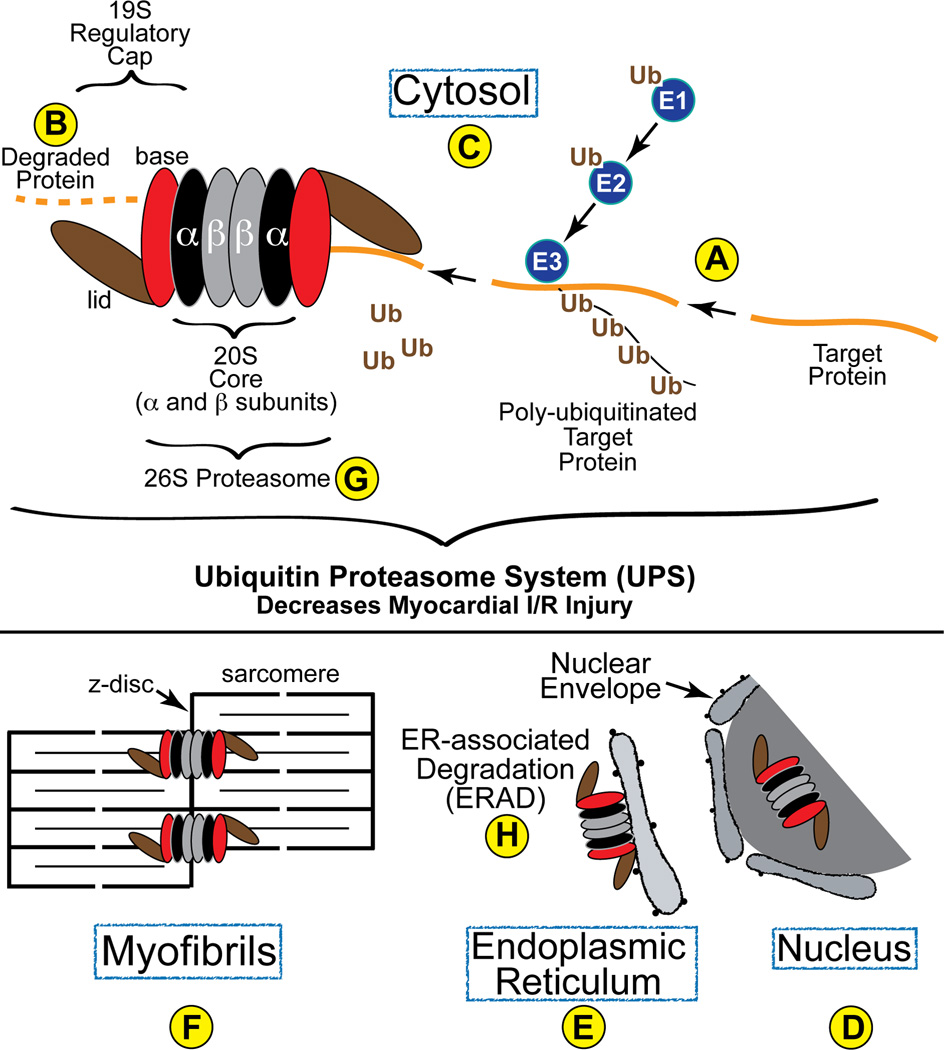
Figure 1. Diagram of the Ubiquitin Proteasome System in the Heart-.
Proteins are targeted for degradation and then ubiquitin is conjugated to them by the concerted transfer of ubiquitin from E1 to E2 and, finally, to E3 ubiquitin ligases, the latter of which can poly-ubiquitinate proteins, as shown (A). Poly-ubiquitinated proteins are then recognized by the 19S regulatory cap of the proteasome, which is composed of a base (red) and a lid (brown). Once within the barrel structure of the 20S portion of the proteasome, which is composed of α- (black) and β-rings (grey), proteins are degraded by the proteolytic activities resident in the β5, β2 and β1 subunits. In cardiac myocytes, proteasomes have been found in the cytosol (C), as well as associated with the z-disc region of sarcomeres (F) and intracellular membranes, such as the SR and ER (E), where they are involved in ERAD (H).