Abstract
Animal models of post traumatic stress disorder (PTSD) are based on fear conditioning where innocuous cues elicit reactions that originally occur to traumatic events – a core feature of PTSD. Another core feature is hyperarousal – exaggerated reactions to stressful events. One limitation of animal models of PTSD is that group effects do not model the sporadic incidence of PTSD. We developed an animal model of PTSD in which rabbit nictitating membrane responses become exaggerated as a function of classical conditioning to a tone conditioned stimulus (CS) paired with a shock unconditioned stimulus (US). Exaggerated responses to the US are a form of hyperarousal termed conditioning-specific reflex modification (CRM) and occur in the absence of the CS. Inspecting data across several experiments, we determined 25% of our rabbits exhibit strong CRM despite all subjects having high levels of conditioning. To determine how prone rabbits were to CRM (susceptibility) or how resistant (resilience), we examined data from 135 rabbits analyzing for factors during CS-US pairings and during US prescreening that would predict CRM. We found the magnitude of CRM was correlated with the onset latency and area of conditioned responding during CS-US pairings and with the peak latency of a response during US pretesting. In an animal model of PTSD that more accurately reflects clinical prevalence, we can begin to predict susceptibility not only during responding to a stressful conditioning situation but also during a screening process before the stressful situation takes place. The results suggest relatively innocuous testing may help detect PTSD after trauma and screen for it before trauma occurs.
Keywords: fear conditioning, nictitating membrane response, post traumatic stress disorder, rabbit, resilience, susceptibility
Introduction
Post-traumatic stress disorder (PTSD) is an anxiety disorder triggered by trauma and symptoms may include re-experiencing, avoidance and hyperarousal. PTSD occurs in 5–15% of those exposed to trauma (Cohen et al., 2004; Kok et al., 2012; McNally, 2012; Ramchand et al., 2010) suggesting there may be factors contributing to a person’s ability to withstand the effects of trauma (resilience) or be prone to them (susceptibility) (Bush et al., 2007; Dudley et al., 2011; Pole et al., 2009; Yehuda & Flory, 2007). Although many traumatic events are unpredictable, in other cases, including combat by frontline troops and responding to accidents and disasters by emergency personnel, trauma could be anticipated and even predicted (Baker et al., 2012; Hourani et al., 2012; Meyer et al., 2012). As a result, there is growing interest in and a need to determine the characteristics of people in and their reaction to predictable traumatic situations to determine resilience and susceptibility (Baker et al., 2012; Meyer et al., 2012). For the majority of cases, however, it is important to understand if and how a person’s reactions to unexpected trauma may help predict PTSD and even prevent it (Pitman et al., 2002; Rothbaum et al., 2012; Zohar et al., 2012).
There are US military programs designed to assess risk (Baker et al., 2012), train resilience (Cornum et al., 2012), and protect troops from PTSD (Morgan & Bibb, 2011). The most obvious risks for military personnel are the number of deployments and exposure to combat during those deployments (Bonanno et al., 2012; Phillips et al., 2010). Resilience in this population is reflected in experience, training, preparedness, positive acceptance of change, belief in fate, availability of secure relationships, and social support (Bonanno et al., 2012; Goldmann et al., 2012; Hourani et al., 2012; Tsai et al., 2012). In civilians, resilience is associated with variables including supportive social networks, resilient role models and spirituality in intensive care unit nurses (Mealer et al., 2012) and social cohesion and mutual support in firefighters (Meyer et al., 2012). Susceptibility on the other hand, has been related to poor social networks, lack of support, occupational stress, childhood abuse, and number of exposures to trauma (Mealer et al., 2012; Meyer et al., 2012; Wrenn et al., 2011). Clearly, understanding factors that increase resilience and reduce susceptibility may help reduce and prevent PTSD and even provide strategies to inoculate against it (Zohar et al., 2012).
Most animal models of PTSD are based on fear conditioning in which innocuous cues elicit reactions that originally occur to stressful events (Cohen et al., 2006; Pawlyk et al., 2005; Pitman et al., 1993; Stam, 2007), but another core feature of PTSD is hyperarousal – exaggerated reactions to stressful events themselves (Siegmund & Wotjak, 2007). We have developed an animal model of PTSD based on observations that rabbit nictitating membrane responses (NMR) and changes in heart rate – different responses to the same aversive event – become exaggerated as a function of classical conditioning (Burhans et al., 2008; Gruart & Yeo, 1995; Schreurs et al., 1995; Schreurs et al., 2000; Schreurs, 2003; Schreurs et al., 2005; Wikgren & Korhonen, 2001). These exaggerated responses occur when the aversive event is tested without the cue and this form of hyperarousal is termed conditioning-specific reflex modification (CRM). CRM is detected by comparing responses to a range of unconditioned stimulus (US) intensities when presented by themselves before and after classical conditioning. CRM is a function of conditioning parameters such as number of conditioned stimulus (CS)-US pairings and US intensity, and it can generalize from one US to another and from one anatomical location to another (Buck et al., 2001; Schreurs et al., 1995; Schreurs et al., 2000; Seager et al., 2003). CRM is also sensitive to context, undergoes extinction, incubation, and spontaneous recovery, is susceptible to drugs and dietary manipulations, and has potential as a model for developing PTSD treatment (Burhans et al., 2010; Schreurs et al., 2005; Schreurs et al., 2006; Schreurs et al., 2007; Schreurs et al., 2011a; Schreurs et al., 2011b; Schreurs et al., 2011c). Conditioning-specific changes in responding to the US characteristic of CRM have been described by several groups in rabbits (Gruart & Yeo, 1995; Schreurs et al., 1995; Wikgren & Korhonen, 2001) and in rats (Servatius et al., 2001). Importantly, Burriss, Ayers, and Powell (2007) have shown that combat veterans with PTSD have eyeblink CRs and URs that are larger than combat veterans without PTSD.
A limitation of animal models of PTSD is that group effects do not model the sporadic incidence of PTSD (Burhans et al., 2008; Cohen et al., 2004; Goswami et al., 2012). Thus, researchers have begun to study individual differences between animals and devised criteria to identify subjects categorized as resilient and susceptible (Burhans et al., 2008; Bush et al., 2007; Cohen et al., 2004; Cohen et al., 2005; Cohen & Zohar, 2004; Goswami et al., 2012; Siegmund & Wotjak, 2006; Stam, 2007; Zohar et al., 2012). Cohen and colleagues have used behavioral cutoffs in sequential tests – elevated plus maze and acoustic startle – to detect maladaptive responses following exposure to predator and immersion stressors (Cohen et al., 2004). They noted that although 90% of subjects initially showed maladaptive responses, only 24% continued to show these responses over time.
We have noted that not all rabbits show CRM despite all having the same high levels of NMR conditioning (Burhans et al., 2008). If CRM is to help model PTSD symptoms, reliance on group data may limit detection of the phenomenon, reducing the opportunity to observe subjects with stress-related changes in behavior considered maladaptive and explore what constitutes susceptibility in some and resilience in others. The purpose of this paper was to analyze NMR conditioning and CRM data from a large number of rabbits trained and tested under identical conditions to identify dependent variable measures from NMR conditioning and US pretesting that correlated with CRM in individual subjects and to determine how these variables might predict CRM. Analysis of NMR conditioning data could help determine how responding during CS-US pairings would anticipate CRM and analysis of US testing data before NMR conditioning could help determine how responding during screening would identify subjects that develop high levels of CRM (susceptible) and those with little, if any, CRM (resilient).
Materials and Methods
Subjects
We analyzed data from 135 rabbits classically conditioned using our standard NMR delay conditioning paradigm. The data came from 70 rabbits in a published study (Schreurs et al., 2011c) and 64 rabbits from two unpublished studies. Subjects were male, New Zealand White rabbits (Oryctolagus cuniculus), supplied by Harlan, weighing 2.0–2.2 kg. Rabbits were housed in individual cages, given free access to food and water, and kept on a 12-hour light/dark cycle. Rabbits were maintained according to NIH guidelines, and the research was approved by the West Virginia University ACUC.
Apparatus
The apparatus and recording procedures are detailed by Schreurs and Alkon (1990) who modeled them after those described by Gormezano (Coleman & Gormezano, 1971; Gormezano, 1966). Each subject was restrained in a Plexiglas box and trained in a sound-attenuating, ventilated chamber (Coulbourn Instruments, Model E10-20). The US was periorbital electrodermal stimulation delivered by a programmable two-pole shocker (Coulbourn Instruments, Model E13-35) via stainless steel wound clips positioned below and posterior to the right eye.
Procedure
All 135 rabbits received one session of adaptation, one 80-trial session of US testing (Pretest), six sessions of paired CS-US presentations, and another 80-trial session of US testing (Post Test). There was only one session presented per day. During the adaptation session, rabbits were prepared for US presentations and recording of the NMR and adapted to training chambers for the duration of subsequent sessions (80 min). On US Pretest and Post Test sessions, rabbits received 80 US trials presented on average every 60 s (50–70 s range). Each trial involved one of 20 combinations of US intensity (0.1, 0.25, 0.5, 1.0, or 2.0 mA) and duration (10, 25, 50, or 100 ms). Four randomized sequences of the 20 combinations were presented on each US testing session and the same intensity or duration did not occur on more than three consecutive trials.
Each conditioning session consisted of 80 presentations of a 400-ms, 1-kHz, 82-dB tone CS co-terminating with a 100-ms, 2.0-mA US (300-ms inter-stimulus interval). Pairings were delivered on average every 60 s (50–70 s range).
A conditioned response (CR) was any nictitating membrane (NM) extension exceeding 0.5 mm initiated after CS onset but before US onset. CR dependent variables were frequency, onset latency, and criterion latency (latency to reach a 0.5-mm response criterion) determined from CS onset to US onset. Over several studies, we noted a strong resemblance between unconditioned response (UR) topographies on US Post Test (CRM) and CR-UR topographies during CS-US pairings, particularly in well-conditioned subjects (Buck et al., 2001; Burhans et al., 2008; Schreurs, 2003). To see how well the full response topography on paired trials predicted UR topography on Post Test, we analyzed peak latency, amplitude and area of a CR from CS onset to the end of the trial (1,800 ms).
A UR was any NM extension exceeding 0.5 mm initiated within 300 ms of US onset. The UR criterion was based on observations that, following CS-US pairings, Post Test URs to lower US intensities had onset latencies that fell within the range of latencies for CRs (Schreurs et al., 2000). Response amplitude was scored in millimeters as maximum NM extension. Response area was total area under the response curve (arbitrary units). Two additional UR measures were calculated to overcome statistical limitations of empty data cells produced by subthreshold responses to the US, particularly at low intensities. These measures – magnitude of response amplitude and magnitude of response area – included amplitudes and areas of all NMRs above baseline (Garcia et al., 2003). All UR data were for five US intensities (0.1, 0.25, 0.5, 1.0, and 2.0 mA) presented across the first 20 trials of US Pretest and Post Test collapsed across US durations (Schreurs, 2003).
Data analysis
We analyzed CR frequency, onset latency, criterion latency, peak latency, amplitude and area during CS-US pairings. CRM measures comprised percent change in UR frequency, magnitude of UR amplitude and magnitude of UR area from Pretest to Post Test. Increases in dependent variable measures from Pretest to Post Test were represented as positive values. If there was no response on Pretest but there was a response on Post Test, percent change was set to 100%. If there was no response on Post Test but there was a response on Pretest, percent change was set to −100%.
To determine the relationship between responding during CS-US pairings and CRM as well as during Pretest and CRM, we calculated correlation coefficients for CR frequency, onset latency, criterion latency, peak latency, amplitude and area versus percent change in UR frequency, magnitude of UR amplitude and magnitude of UR area. To measure the degree of relationship between these sets of CR performance variables and the CRM predicted variables we calculated Canonical Correlations using SYSTAT (Crane Software). Canonical correlations are multivariate statistics describing interrelationships among sets of variables (Harris, 2001; Sherry & Henson, 2005) particularly when there are multiple dependent variables ( measures of CRM) and multiple independent variables (measures of CR performance, measures of URs on US Pretest). An overall association between variables is expressed as R2 (−1.0 ≤ R2 ≤ 1.0) and significance determined by a specific probability (PROB). We use the canonical correlations as an omnibus test for significance among the multiple dependent and independent variables and bivariate correlations to explore the specific relationships.
Results
NMR Conditioning
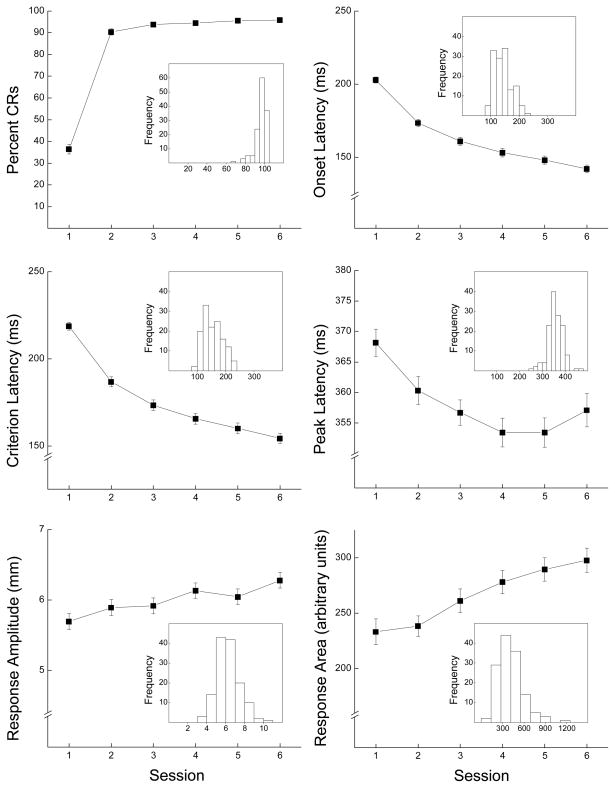
Six panels in Figure 1 depict CR frequency, onset latency, criterion latency, peak latency, amplitude and area across six sessions of CS-US pairings. The inset in each panel illustrates the frequency distribution of each dependent variable on the last session of CS-US pairings. The top left panel shows all rabbits acquired CRs within two days of pairings and reached asymptotic levels greater than 95% CRs (range: 83% to 100%). The remaining panels show CR onset, criterion and peak latencies decreased across sessions and response amplitude and area increased. The insets show relatively tight frequency distributions as the error bars would suggest. Analyses of variance (ANOVAs) yielded significant effects of days of CS-US pairings for all six dependent variables, (F’s (5,670) > 7.60, p’s < .001).
Figure 1.
The six panels depict conditioned response (CR) frequency (Percent CRs), onset latency (ms), criterion latency (ms), peak latency (ms), amplitude (mm) and area (arbitrary units) across six sessions of conditioned stimulus-unconditioned stimulus pairings. The inset for each panel shows the frequency distribution for the conditioned response measure during the sixth session of conditioned stimulus-unconditioned stimulus pairings. The conditioned stimulus was a 400-ms, 1-kHz, 82-dB tone paired with a 100-ms, 2.0-mA periorbital electrodermal unconditioned stimulus (300-ms inter-stimulus interval).
CRM
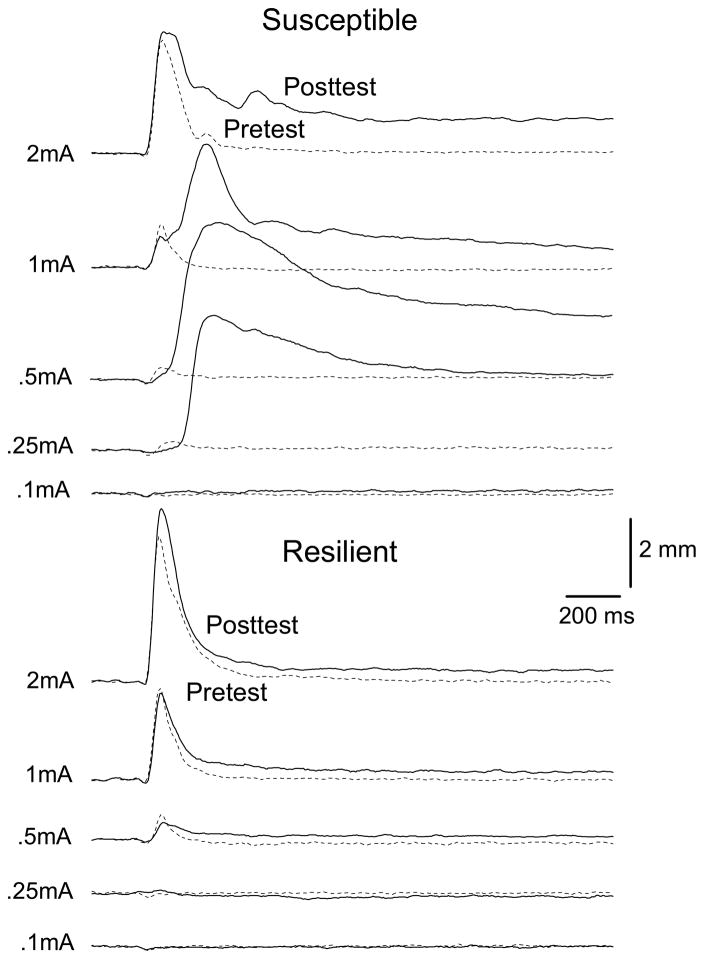
Individual subject Post Test data indicate CRM is not universal and although some rabbits show large changes in responding from Pretest to Post Test, others show little, if any, changes (Burhans et al., 2008). Figure 2 shows responding to five US intensities by two subjects trained and tested at the same time where the top rabbit (Susceptible) shows profound increases in response amplitude (1200%) and area (2000%) particularly at intermediate US intensities (0.25 and 0.5 mA) following CS-US pairings (Post Test) and the bottom rabbit (Resilient) shows very little change in amplitude (−80%) or area (−80%). These differences exist despite high levels of CR acquisition for both subjects (100% vs. 98.7% CRs).
Figure 2.
Response topographies to five unconditioned stimulus (US) intensities on Pretest and Post Test by two subjects trained and tested at the same time. Responses are for the first 20 US presentations and averaged across four US durations. The top rabbit (Susceptible) shows substantial increases in response amplitude and area particularly at intermediate US intensities (0.25 and 0.5 mA) following conditioned stimulus-unconditioned stimulus pairings (Post Test) and the bottom rabbit (Resilient) shows very little change in responding from Pretest to Post Test. Both rabbits had high levels of conditioned response (CR) acquisition (100% vs. 98.7% CRs).
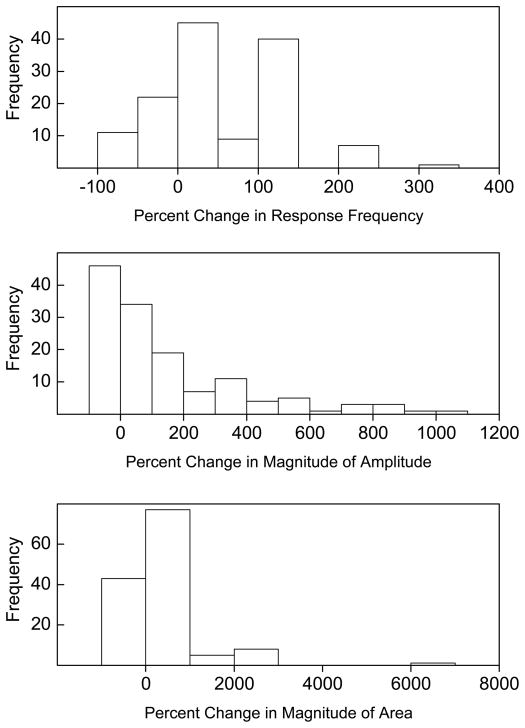
Figure 3 graphs the frequency distribution of all rabbits as a function of the percent change in UR frequency, magnitude and magnitude of response area collapsed across the five US intensities. The figure shows a very wide range of increases in the three measures of CRM for most of the 135 rabbits as well as a minority of rabbits that showed a Pretest to Post Test decrease. To obtain an estimate of the number of rabbits showing CRM, we calculated mean and standard deviation of UR frequency, magnitude of response amplitude, and magnitude of response area on Pretest and compared this to each individual subjects’ Post Test values. A total of 77 rabbits (57%) had at least one dependent variable indicative of CRM – defined as a Post Test value that was at least one standard deviation greater than the Pretest mean. To obtain a more stringent estimate of the number of rabbits showing CRM, we calculated mean and standard deviation of percent change from Pretest to Post Test in frequency, magnitude of response amplitude, and magnitude of response area. This analysis takes into account that many rabbits show some level of CRM but focuses on rabbits that had the strong levels seen in Figure 2. That is, we selected subjects with abnormally large reactions to stress – the central tenet of PTSD. A total of 47 rabbits (34.8%) had at least one measure of CRM that was one standard deviation above the mean percent change: 15.7% had UR frequencies, 26.1% had magnitudes of response amplitude, and 11.2% had magnitudes of response area greater than one standard deviation above the mean. Given individual differences in the presence and level of CRM despite comparably high levels of CR acquisition, we determined if there were behavioral variables measured during CS-US pairings that correlated with CRM and might be predictive of it.
Figure 3.
The frequency distribution of rabbits for the percent change from Pretest to Post Test for unconditioned response frequency, magnitude (of amplitude, mm) and magnitude of response area (arbitrary units) collapsed across unconditioned stimulus intensities.
CRM and NMR Conditioning
We compared measures of CR frequency, onset latency, criterion latency, peak latency, amplitude and area for each session of CS-US pairings (predictors) with CRM measures of percent change in UR frequency and percent change in magnitude of UR amplitude and magnitude of UR area. Multivariate canonical correlation analyses revealed significant relationships between CR area and magnitude of UR amplitude (R2 = 0.343, PROB = 0.005) and magnitude of UR area (R2 = 0.315, PROB = 0.018), between CR amplitude and magnitude of UR area (R2 = 0.335, PROB = 0.007), and CR onset latency and magnitude of UR amplitude (R2 = 0.322, PROB = 0.013). We then examined univariate correlation coefficients for the predictors identified by the canonical correlations (CR area and onset latency) and measures of CRM. The correlation coefficients are listed in Table 1. There were a number of significant positive univariate correlations between CR area and percent change in UR frequency, magnitude of UR amplitude, and magnitude of UR area by the last session of CS-US pairings but as early as the second session (0.17 < r’s < .38, 0.05 < p’s < .0001) suggesting the larger the CR the larger the CRM. The largest of these correlations were for magnitude of UR amplitude at 0.25 mA (r=0.38, p < .0001) and 0.5 mA (r=0.31, p < .001) where we see the largest levels of CRM (Susceptible, Figure 2). There were significant negative correlations between CR latency measures of onset and UR frequency, magnitude of UR amplitude, and magnitude of UR area (−0.17 < r’s < −.28, 0.05 < p’s < .0001) that began to emerge by the third session of CS-US pairings but were largest on the last session. The negative latency correlations indicate the shorter the onset latency with which rabbits responded to the CS during CS-US pairings the greater the magnitude of CRM.
Table 1.
Correlation coefficients of conditioned response area and conditioned response onset latency for percent change in unconditioned response frequency, magnitude and magnitude of area for unconditioned stimulus intensities of 0.1, 0.25, 0.5, 1.0 and 2.0 mA for each of six sessions of conditioned stimulus-unconditioned stimulus pairings
| Conditioned Response Area | Conditioned Response Onset Latency | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 mA | 0.25 mA | 0.5 mA | 1 mA | 2 mA | 0.1 mA | 0.25 mA | 0.5 mA | 1 mA | 2 mA | ||
| Percent Change | |||||||||||
| Frequency | Session 1 | 0.06 | 0.07 | 0.12 | 0.1 | 0.06 | 0.00 | −0.08 | 0.05 | 0.08 | −0.08 |
| Session 2 | −0.07 | 0.01 | 0.11 | 0.05 | 0.10 | 0.00 | −0.12 | −0.15 | −0.24 ** | −0.07 | |
| Session 3 | 0.08 | 0.13 | 0.15 | 0.04 | 0.17 * | −0.03 | −0.13 | −0.16 | −0.18 * | −0.02 | |
| Session 4 | 0.03 | 0.18 * | 0.25 ** | −0.02 | 0.19 * | −0.02 | −0.15 | −0.15 | −0.13 | −0.07 | |
| Session 5 | −0.07 | 0.10 | 0.22 * | −0.02 | 0.26 ** | −0.01 | −0.15 | −0.23 ** | −0.12 | −0.06 | |
| Session 6 | −0.08 | 0.16 | 0.21 * | 0.10 | 0.23 ** | −0.02 | −0.20 * | −0.23 ** | −0.23 ** | −0.13 | |
| Magnitude | Session 1 | 0.10 | 0.06 | 0.13 | 0.12 | 0.09 | −0.19 | −0.12 | 0.07 | −0.12 | −0.06 |
| Session 2 | 0.04 | 0.17 * | 0.11 | 0.17 * | 0.12 | −0.10 | −0.07 | −0.09 | −0.09 | −0.16 | |
| Session 3 | 0.03 | 0.29 *** | 0.18 * | 0.18 * | 0.16 | −0.09 | −0.20 * | −0.17 * | −0.13 | −0.17 * | |
| Session 4 | 0.02 | 0.38 *** | 0.31 *** | 0.20 * | 0.22 * | −0.11 | −0.19 * | −0.17 ** | −0.13 | −0.23 ** | |
| Session 5 | 0.04 | 0.25 ** | 0.26 ** | 0.16 | 0.22 * | −0.01 | −0.15 | −0.23 ** | −0.10 | −0.13 | |
| Session 6 | −0.08 | 0.32 *** | 0.24 ** | 0.27 ** | 0.13 | −0.05 | −0.26 ** | −0.28 *** | −0.18 * | −0.20 * | |
| Magnitude of Area | Session 1 | −0.09 | 0.11 | 0.17 * | 0.16 | 0.11 | −0.04 | −0.08 | −0.03 | −0.12 | −0.08 |
| Session 2 | −0.03 | 0.24 ** | 0.13 | 0.13 | 0.15 | 0.06 | −0.12 | −0.11 | −0.02 | −0.02 | |
| Session 3 | −0.09 | 0.19 * | 0.16 | 0.17 * | 0.29 *** | 0.13 | −0.16 | −0.12 | −0.10 | −0.06 | |
| Session 4 | −0.10 | 0.19 * | 0.07 | 0.22 * | 0.37 *** | 0.13 | −0.16 | −0.11 | −0.11 | −0.07 | |
| Session 5 | −0.10 | 0.13 | 0.09 | 0.18 * | 0.26 ** | 0.15 | −0.14 | −0.09 | −0.12 | −0.05 | |
| Session 6 | −0.08 | 0.19 * | 0.22 * | 0.21 * | 0.30 *** | 0.05 | −0.18 * | −0.11 | −0.13 | −0.09 | |
p < .05,
p < .01,
p < .001
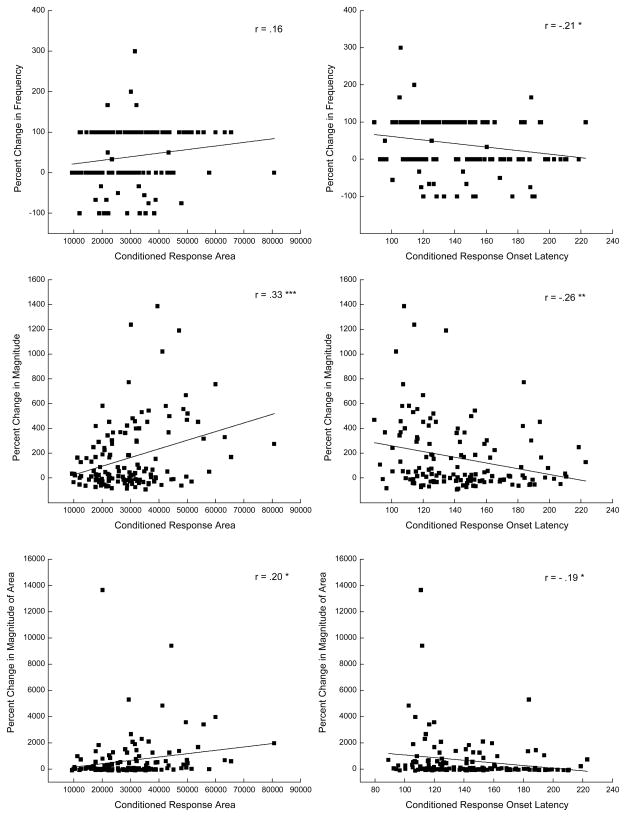
Figure 4 provides scatter plots of the correlation data for each of the measures of CRM at a single US intensity (0.25 mA) for area and onset latencies of CRs that occurred on the sixth session of CS-US pairings. In comparison to Table 1, the scatter plots do not illustrate the highest correlations but are included to show the data for a single US intensity on a single day that depict a number of significant positive and negative correlations as well as a non-significant correlation. The scatter plots illustrate a number of important points. Looking at the top two plots, the majority of rabbits responded to the US on both Pretest and Post Test (0 percent change) or did not respond on Pretest and did respond on Post Test (100 percent change). The middle plots show the strongest correlations and that a number of rabbits showed increases in the magnitude of responding to the 0.25-mA US on Post Test of 200% or more. The right scatter plot illustrates quite clearly that the largest changes in magnitude were for rabbits that had some of the shortest CR onset latencies. Finally, the bottom two plots show that only a very few rabbits had extremely large increases in the magnitude of the area of their responses and that these increases were an order of magnitude larger that the increases in response magnitude. Nevertheless, there were still a number of rabbits that had increases in the magnitude of the area of up to 2000 percent confirming that rabbits were susceptible to the effects of CS-US pairings. These scatter plots are also representative of the plots obtained for responding at 0.5 mA on the last session of CS-US pairings and at both 0.25 and 0.5 mA on earlier sessions of CS-US pairings.
Figure 4.
Scatter plots of correlation data in Table 1 with a line of best linear fit for conditioned response area and conditioned response onset latency (ms) at a single unconditioned stimulus intensity (0.25 mA) versus percent change in unconditioned response frequency, magnitude, and magnitude of the area that occurred on the sixth session of conditioned stimulus-unconditioned stimulus pairings (Day 6).
To determine whether we could reliably detect differences in performance during CS-US pairings for rabbits considered to be highly susceptible, we conducted ANOVAs of CR frequency, onset latency, amplitude, peak latency and area for rabbits at least one standard deviation above the mean percent change in CRM and considered susceptible compared to those that were not susceptible. Given the same levels of CR acquisition for all rabbits, there were no effects of susceptibility for CR frequency (F’s < 2.66, p’s > .1). In contrast, there were main effects of susceptibility for CR onset latency (F(1, 133) = 5.77, p < .05) and area (F(1, 133) = 10.52, p < .005), and interactions of susceptibility with days of CS-US pairings for CR onset latency (F(5, 665) = 4.59, p < .001), amplitude (F(5, 665) = 2.63, p < .05), and area (F(5, 665) = 4.59, p < .001). As suggested by the correlations, these data confirm that although susceptible rabbits had the same level of CRs as those that showed little, if any, CRM, CRs were larger and occurred with shorter latencies. These effects emerged as a function the number of CS-US pairings.
CRM and Pretest
As important as it is to determine if PTSD will occur based on a person’s response to trauma, there would be significant advantages to screening for the likelihood of PTSD before trauma was encountered. We calculated correlations between US responding on US Pretest and measures of CRM (UR frequency, magnitude of UR amplitude and magnitude of UR area). Given the percent change scores used to assess CRM are dependent on the Pretest values used to calculate them, only correlations between UR Pretest and CRM measures independent of each other were considered (onset and peak latencies versus change in UR frequency, magnitude of UR amplitude and magnitude of UR area). In fact, analyses of measures that were dependent particularly Pretest UR frequency and percent change in UR frequency yielded a very high canonical correlation (R2 = 0.977, PROB = 0.000) as one would expect where one measure contains elements of the other as well as a highly significant difference between subjects that would be considered susceptible and those considered resilient (F’s > 7, p’s < .0001).
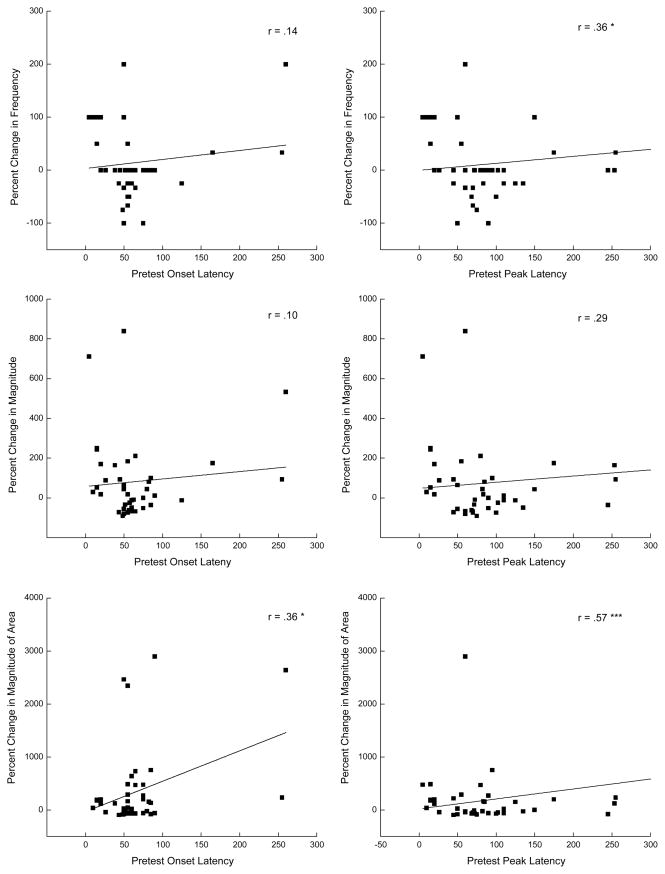
Canonical correlation analyses revealed a significant relationship between Pretest peak latency and percent change in magnitude of UR amplitude (R2 = 0.238, PROB = 0.021). Table 2 lists individual correlations and shows peak latencies to a 0.25-mA Pretest US were significantly related to percent change in UR frequency (r=0.36, p < .05) and magnitude of UR area (r=0.57, p < .001) to a 0.5-mA US. A similar but less striking effect was noted for onset latency and magnitude of UR area (r’s=.36, p’s < .05). These positive correlations are based on the 41 rabbits that responded to the 0.25-mA US on Pretest and the data for responding at 0.25 mA on Pretest versus percent changes in UR frequency, magnitude of UR amplitude and magnitude of UR area at 0.5 mA are depicted as scatter plots in Figure 5. The scatter plots show Pretest peak latencies are more spread than the onset latencies and, looking specifically at the bottom two plots, the majority of rabbits show an increase of up to 1000% in the magnitude of UR area. Taken together, the data imply that the later the UR onset and peak occurred to a weak shock on Pretest, the more likely there were to be changes in the likelihood and size of CRM at US values where CRM is most often detected (Burhans et al., 2008; Schreurs, 2003).
Table 2.
Correlation coefficients of Pretest unconditioned response onset latency and peak latency for percent change in unconditioned response frequency, magnitude and magnitude of area for unconditioned stimulus intensities of 0.1, 0.25, 0.5, 1.0 and 2.0 mA.
| Pretest Onset Latency | Pretest Peak Latency | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 mA | 0.25 mA | 0.5 mA | 1 mA | 2 mA | 0.1 mA | 0.25 mA | 0.5 mA | 1 mA | 2 mA | ||
| Percent Change | |||||||||||
| Frequency | 0.1 mA | −0.14 | 0.20 | 0.00 | −0.12 | −0.30*** | −0.14 | 0.05 | −0.18 | −0.16 | −0.30*** |
| 0.25 mA | 0.09 | 0.04 | 0.17 | −0.09 | −0.12 | −0.16 | −0.01 | −0.12 | −0.06 | −0.05 | |
| 0.5 mA | 0.00 | 0.14 | 0.20 * | 0.15 | 0.01 | −0.02 | 0.36 * | 0.03 | −0.03 | −0.10 | |
| 1 mA | 0.42 | 0.09 | 0.18 | 0.32 *** | 0.15 | 0.17 | 0.10 | 0.13 | 0.00 | 0.05 | |
| 2 mA | −0.16 | 0.36 * | 0.00 | −0.12 | 0.00 | −0.08 | 0.10 | 0.04 | −0.04 | 0.00 | |
| Magnitude | 0.1 mA | −0.02 | 0.02 | −0.14 | −0.08 | −0.14 | −0.23 | 0.03 | −0.14 | −0.03 | −0.12 |
| 0.25 mA | −0.06 | −0.02 | 0.08 | −0.11 | −0.03 | −0.09 | −0.12 | −0.05 | −0.11 | −0.03 | |
| 0.5 mA | −0.18 | 0.10 | 0.21 * | 0.11 | 0.07 | −0.05 | 0.29 | −0.08 | −0.05 | −0.02 | |
| 1 mA | 0.13 | 0.17 | 0.10 | 0.19 * | 0.08 | 0.20 | 0.34 * | 0.02 | −0.14 | −0.03 | |
| 2 mA | 0.08 | 0.35 * | 0.05 | 0.10 | 0.12 | 0.13 | 0.38 * | 0.23 * | 0.11 | −0.08 | |
| Magnitude of Area | 0.1 mA | 0.01 | 0.10 | −0.05 | −0.07 | −0.06 | −0.18 | 0.28 | −0.08 | −0.05 | −0.02 |
| 0.25 mA | 0.05 | 0.06 | −0.04 | −0.08 | −0.07 | −0.05 | −0.10 | −0.05 | −0.05 | 0.05 | |
| 0.5 mA | −0.10 | 0.36 * | 0.11 | 0.02 | 0.00 | −0.10 | 0.57 *** | −0.11 | −0.08 | 0.06 | |
| 1 mA | 0.06 | 0.13 | 0.04 | 0.04 | 0.05 | 0.00 | 0.31 * | −0.03 | −0.14 | −0.05 | |
| 2 mA | 0.02 | 0.37 * | 0.07 | 0.07 | 0.00 | −0.10 | 0.46 ** | −0.05 | −0.11 | −0.27 ** | |
p < .05,
p < .01,
p < .001
Figure 5.
Scatter plots of correlation data in Table 2 with a line of best linear fit for unconditioned response onset and peak latency (ms) for the 41 rabbits that responded to an unconditioned stimulus intensity of 0.25 mA at Pretest versus percent change in unconditioned response frequency, magnitude, and magnitude of the area at 0.5 mA. * p < .05, ** p < .01.
To determine whether we could detect differences in responding during Pretest for rabbits that would be considered susceptible, we conducted ANOVAs on UR frequency, onset, and peak latency for rabbits that were one standard deviation above the mean percent change in CRM and those that were not. Repeated measures analyses do not tolerate missing data and the low levels of responding to US intensities of 0.1 and .25 mA required analyses to be restricted to US intensities of 0.5, 1.0, and 2.0 mA. With this restriction, 26 subjects were deleted due to missing data. Given the reliance of CRM measures on Pretest UR frequency, there were significant effects of susceptibility for UR frequency (F’s > 7, p’s < .0001). Importantly, there was also main effects of susceptibility for UR onset latency (F(1, 106) = 5.08, p < .05) and an interaction of susceptibility with Pretest US intensity for UR onset latency (F(2, 212) = 3.16, p < .05), but not peak latency (F’s < 1.6, p’s > .22). However, these Pretest data may be less sensitive to change because of the large amount of missing data – data where we see many of our most significant changes (Figure 2). Analyses of Pretest UR measures that included responses to a 0.25 mA US resulted in 98 deleted cases but did yield significant effects of peak latency (F(3, 105) = 4.45, p < .01) and onset latency (F’s > 3.02, p’s < .05). As suggested by the correlations, these data confirm susceptible rabbits had Pretest URs that began and peaked more slowly than rabbits that showed little, if any, CRM.
Discussion
The principal finding of these analyses was that size of CRM was correlated with latency and area of CRs during CS-US pairings and with peak latency of URs during a Pretest session administered before CS-US pairings. Specifically, CRM occurred in rabbits with CRs that had shorter onset latencies and had larger areas during training and in rabbits whose URs had longer peak latencies during testing before training. These data came from a large group of animals trained and tested under identical conditions in numerous replications over many months and suggest, in an animal model of PTSD, we can begin to predict susceptibility to CRM not only during responding to a stressful conditioning situation but also during a screening process that occurs before the stressful situation takes place.
We have previously noted the topography of a UR after CS-US pairings bears a striking resemblance to the CR-UR sequence that occurs during CS-US pairings, not only for the NMR (Schreurs et al., 2000) but for heart rate as well (Burhans et al., 2010; Schreurs et al., 2005; Schreurs et al., 2011a). That is not to say that CRM is simply a CR elicited by weak shocks after training because extinguishing CRs does not extinguish CRM and extinguishing CRM does not extinguish CRs (Schreurs et al., 2000). Moreover, there is no evidence rabbits can generalize from tones to lights let alone from tones to shocks (Kehoe & Holt, 1984; Kehoe & Napier, 1991; Schreurs & Kehoe, 1987). Nevertheless, the significant correlation between CR area and CRM confirms there is a relationship between the two responses. As briefly described in the introduction, an eyeblink conditioning study in veterans by Burris and colleagues showed combat veterans with PTSD had larger CRs and URs than combat veterans and non-combat veterans without PTSD (Burriss et al., 2007). Given the neural pathways for eyeblink conditioning in humans and rabbits show considerable similarity, the correspondence between PTSD and CRM suggests eyeblink conditioning and CRM may provide insights about the biological basis of PTSD. For example, the amygdala has long been implicated in classical conditioning in both humans (Linnman et al., 2011; Marschner et al., 2008) and rabbits (Kim & Jung, 2006; Powell et al., 1997) as well as in PTSD (Robinson & Shergill, 2011; Sripada et al., 2012) and we have shown that infusions of the GABA agonist muscimol into the amygdala affects the expression of CRM (Burhans & Schreurs, 2008).
The significant negative correlations between CR latency and CRM is intriguing. These results bear some similarity to reaction times shown to change as a function of PTSD with evidence reaction time to a neutral word is faster if it follows a threat in PTSD patients (Amir et al., 2009) and neural activity in PTSD patients is faster to threat stimuli with reactions becoming faster the more severe the trauma (Rockstroh & Elbert, 2010). This is not unequivocal however, because there is also evidence of longer reaction times and longer evoked-response potentials as a result of PTSD (Hennig-Fast et al., 2009).
Also intriguing is the fact that CRM was correlated with peak latency on Pretest. The data indicate the longer a response takes to reach its peak to a weak US on Pretest the more likely a rabbit will show CRM following CS-US pairings. As important as it is to be able to predict PTSD from a person’s reaction to the trauma (Zohar et al., 2012), the ability to screen for someone susceptible to PTSD is important to those exposed to trauma on a regular basis including combat troops, first responders, and other emergency personnel (Baker et al., 2012; Hourani et al., 2012; Meyer et al., 2012). The present peak latency results indicate we might be able to screen for CRM based on the timing of response peaks to weak shocks. This has clinical relevance because these US test intensities are weak enough to only elicit a minimal response and therefore, are not very aversive. As such, weak shocks could be an effective screen for CRM without having to present a range of US intensities during Pretest, particularly higher intensities.
Because it normally takes several days of classical conditioning before exaggerated responses emerge (Schreurs et al., 1995), CRM begins to approximate the repeated exposure to stress that occurs in situations that can result in PTSD including extended tours of duty in a combat zone, physically or sexually abusive relationships, or living through a disaster (Kaysen et al., 2003; Miller et al., 2007). This is a model of chronic exposure to stress rather than the single traumatic event thought by some to be necessary for a diagnosis PTSD (Friedman et al., 2011).
We have shown elsewhere that unpaired presentations of a weak US (0.25 mA) and a tone CS are capable of extinguishing both CRs and CRM (Schreurs et al., 2011c). In a clinical setting this means presentations of stressful but not traumatic stimuli could be used to reduce hyperarousal experienced by patients with PTSD as well as reactions to the sights and sounds associated with trauma (Schreurs et al., 2011c). For example, there are virtual reality programs that could incorporate strong trauma-associated stimuli into a virtual environment (McLay et al., 2012; Reger et al., 2011) that could include mild electrical shocks to the skin as part of a treatment protocol. The present results suggest by monitoring reactions to a traumatic situation, virtual reality treatments could be instituted immediately after trauma for individuals who show aberrant reaction times to treat them for the disorder (Searcy et al., 2012; Zohar et al., 2012). The results also suggest screening for reaction times in a virtual reality environment that mimics a potentially traumatic environment like a war zone or disaster site before people are exposed to those situations could help select for those susceptible to PTSD (Rizzo et al., 2012).
Acknowledgments
Preparation of the manuscript and the experiments described was supported by the National Institutes of Health, National Institute of Mental Health (MH081159). The contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the National Institute of Mental Health.
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or conflicts of interest.
Reference List
- Amir N, Taylor CT, Bomyea JA, Badour CL. Temporal allocation of attention toward threat in individuals with posttraumatic stress symptoms. Journal of Anxiety Disorders. 2009;23:1080–1085. doi: 10.1016/j.janxdis.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Nash WP, Litz BT, Geyer MA, Risbrough VB, Nievergelt CM, et al. Predictors of risk and resilience for Posttraumatic Stress Disorder among ground combat marines: methods of the Marine Resiliency Study. Preventing Chronic Disease. 2012;9:110134. doi: 10.5888/pcd9.110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA, Mancini AD, Horton JL, Powell TM, LeardMann CA, Boyko EJ, et al. Trajectories of trauma symptoms and resilience in deployed U.S. military service members: prospective cohort study. British Journal of Psychiatry. 2012;200:317–323. doi: 10.1192/bjp.bp.111.096552. [DOI] [PubMed] [Google Scholar]
- Buck DL, Seager MA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: generality and nature of the phenomenon. Behavioral Neuroscience. 2001;115:1039–1047. [PubMed] [Google Scholar]
- Burhans LB, Schreurs BG. Inactivation of the central nucleus of the amygdala abolishes conditioning-specific reflex modification of the rabbit nictitating membrane response and delays classical conditioning. Behavioral Neuroscience. 2008;122:75–88. doi: 10.1037/0735-7044.122.1.75. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit’s nictitating membrane response and heart rate: behavioral rules, neural substrates, and potential applications to post-traumatic stress disorder. Behavioral Neuroscience. 2008;122:1191–1206. doi: 10.1037/a0013599. [DOI] [PubMed] [Google Scholar]
- Burhans LB, Smith-Bell CA, Schreurs BG. Effects of extinction on classical conditioning and conditioning-specific reflex modification of rabbit heart rate. Behavioural Brain Research. 2010;206:127–134. doi: 10.1016/j.bbr.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burriss L, Ayers E, Powell DA. Combat veterans show normal discrimination during differential trace eyeblink conditioning, but increased responsivity to the conditioned and unconditioned stimulus. Journal of Psychiatric Research. 2007;41:785–794. doi: 10.1016/j.jpsychires.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Bush DEA, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. Journal of Traumatic Stress. 2007;20:413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Richter-Levin G, Zohar J. The contribution of an animal model toward uncovering biological risk factors for PTSD. Annals New York Academy of Sciences. 2006;1071:335–350. doi: 10.1196/annals.1364.026. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J. An animal model of posttraumatic stress disorder. The use of cut-off behavioral criteria. Annals New York Academy of Sciences. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Kaplan Z, Geva AB. Unsupervised fuzzy clustering analysis supports behavioral cutoff criteria in an animal model of posttraumatic stress disorder. Biological Psychiatry. 2005;58:640–650. doi: 10.1016/j.biopsych.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- Coleman SR, Gormezano I. Classical conditioning of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response under symmetrical CS-US interval shifts. Journal of Comparative and Physiological Psychology. 1971;77:447–455. doi: 10.1037/h0031879. [DOI] [PubMed] [Google Scholar]
- Cornum R, Matthews MD, Seligman MEP. Comprehensive Soldier Fitness: building resilience in a challenging institutional context. American Psychologist. 2012;66:4–9. doi: 10.1037/a0021420. [DOI] [PubMed] [Google Scholar]
- Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neuroscience and Biobehavioral Reviews. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depression and Anxiety. 2011;28:737–749. doi: 10.1002/da.20845. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD, Weidemann G, Kehoe EJ. Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behavioral Neuroscience. 2003;117:292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Goldmann E, Calabrese JR, Prescott MR, Tamburrino M, Liberzon I, Slembarski R, et al. Potentially modifiable pre-, peri-, and postdeployment characteristics associated with deployment-related posttraumatic stress disorder among Ohio army national guard soldiers. Annals of Epidemiology. 2012;22:71–78. doi: 10.1016/j.annepidem.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental methods and instrumentation in psychology. New York: McGraw-Hill; 1966. pp. 385–420. [Google Scholar]
- Goswami S, Samuel S, Sierra OR, Cascardi M, Pare D. A rat model of post-traumatic stress disorder reproduces the hippocampal deficits seen in the human syndrome. Frontiers in Behavioral Neuroscience. 2012;6 doi: 10.3389/fnbeh.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Experimental Brain Research. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Harris RJ. A primer of multivariate statistics. 3. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 2001. [Google Scholar]
- Hennig-Fast K, Werner NS, Lermer R, Latscha K, Meister F, Reiser M, et al. After facing traumatic stress: brain activation, cognition and stress coping in policemen. Journal of Psychiatric Research. 2009;43:1146–1155. doi: 10.1016/j.jpsychires.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Hourani L, Bender R, Weimer B, Peeler R, Bradshaw M, Lane MB, et al. Longitudinal study of resilience and mental health in marines leaving military service. Journal of Affective Disorders. 2012;139:154–165. doi: 10.1016/j.jad.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Kaysen D, Resick PA, Wise D. Living in danger: the impact of chronic traumatization and the traumatic context on posttraumatic stress disorder. Trauma, Violence, & Abuse. 2003;4:247–264. doi: 10.1177/1524838003004003004. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Holt PE. Transfer across CS-US intervals and sensory modalities in classical conditioning of the rabbit. Animal Learning & Behavior. 1984;12:122–128. [Google Scholar]
- Kehoe EJ, Napier RM. Temporal specificity in cross-modal transfer of the rabbit nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:26–35. [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok BC, Herrell RK, Thomas JL, Hoge CW. Posttraumatic stress disorder associated with combat service in Iraq or Afghanistan. Reconciling prevalence differences between studies. Journal of Nervous and Mental Disease. 2012;200:444–450. doi: 10.1097/NMD.0b013e3182532312. [DOI] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behavioural Brain Research. 2011;2221:237–245. doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervleit B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. Journal of Neuroscience. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Graap K, Spira J, Perlman K, Johnston S, Rothbaum BO, et al. Development and testing of virtual reality exposure therapy for post-traumatic stress disorder in active duty service members who served in Iraq and Afghanistan. Military Medicine. 2012;177:635–642. doi: 10.7205/milmed-d-11-00221. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Are we winning the war against Posttraumatic Stress Disorder? Science. 2012;336:872–874. doi: 10.1126/science.1222069. [DOI] [PubMed] [Google Scholar]
- Mealer M, Jones J, Moss M. A qualitative study of resilience and posttraumatic stress disorder in United States ICU nurses. Intensive Care Medicine. 2012 doi: 10.1007/s00134-012-2600-6. in press. [DOI] [PubMed] [Google Scholar]
- Meyer EC, Zimering R, Daly E, Knight J, Kamholz BW, Gulliver SB. Predictors of posttraumatic stress disorder and other psychological symptoms in trauma-exposed firefighters. Psychological Services. 2012;9:1–15. doi: 10.1037/a0026414. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Bibb SC. Assessment of military population-based psychological resilience programs. Military Medicine. 2011;176:976–985. doi: 10.7205/milmed-d-10-00433. [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbance in posttraumatic stress disorder: the role of context after fear conditioning. Biological Psychiatry. 2005;57:268–277. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Phillips CJ, LeardMann CA, Gumbs GR, Smith B. Risk factors for posttraumatic stress disorder among deployed US male marines. BMC Psychiatry. 2010;10:52. doi: 10.1186/1471-244X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Shalev AY. Once bitten, twice shy: beyond the conditioning model of PTSD. Biological Psychiatry. 1993;33:145–146. doi: 10.1016/0006-3223(93)90132-w. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biological Psychiatry. 2009;65:235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Chachich M, Murphy V, McLaughlin J, Tebbutt D, Buchanan SL. Amygdala-prefrontal interactions and conditioned bradycardia in the rabbit. Behavioral Neuroscience. 1997;111:1056–1074. doi: 10.1037//0735-7044.111.5.1056. [DOI] [PubMed] [Google Scholar]
- Ramchand R, Schell TL, Karney BR, Osilla KC, Burns RM, Caldarone LB. Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: possible explanations. Journal of Traumatic Stress. 2010;23:59–68. doi: 10.1002/jts.20486. [DOI] [PubMed] [Google Scholar]
- Reger GM, Holloway KM, Candy C, Rothbaum BO, Difede J, Rizzo AA, et al. Effectiveness of virtual reality exposure therapy for active duty soldiers in a military mental health clinic. Journal of Traumatic Stress. 2011;24:93–96. doi: 10.1002/jts.20574. [DOI] [PubMed] [Google Scholar]
- Rizzo A, Buckwalter JG, John B, Newman B, Parsons T, Kenny P, et al. STRIVE: Stress Resilience In Virtual Environments: a pre-deployment VR system for training emotional coping skills and assessing chronic and acute stress responses. Studies in Health Technology and Informatics. 2012;173:379–385. [PubMed] [Google Scholar]
- Robinson BL, Shergill SS. Imaging in posttraumatic stress disorder. Current Opinion in Psychiatry. 2011;24:29–33. doi: 10.1097/YCO.0b013e3283413519. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Elbert T. Traces of fear in the neural web--magnetoencephalographic responding to arousing pictorial stimuli. International Journal of Psychophysiology. 2010;78:14–19. doi: 10.1016/j.ijpsycho.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, et al. Early intervention may prevent the development of posttraumatic stress disorder: a random pilot civilian study with modified prolonged exposure. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.002. http://dx.doi.org/10.1016/j.biopsych.2012.06.002. [DOI] [PMC free article] [PubMed]
- Schreurs BG. Classical conditioning and modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Behavioral and Cognitive Neuroscience Reviews. 2003;2:83–96. doi: 10.1177/1534582303255014. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Crum JM, Wang D, Smith-Bell CA. Conditioning-specific reflex modification of rabbit (Oryctolagus cuniculus) heart rate. Behavioral Neuroscience. 2005;119:1484–1495. doi: 10.1037/0735-7044.119.6.1484. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gonzalez-Joekes J, Smith-Bell CA. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response is sensitive to context. Learning & Behavior. 2006;34:315–324. doi: 10.3758/bf03192886. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Kehoe EJ. Cross-modal transfer as a function of initial training level in classical conditioning with the rabbit. Animal Learning & Behavior. 1987;15:47–54. [Google Scholar]
- Schreurs BG, Oh MM, Hirashima C, Alkon DL. Conditioning-specific modification of the rabbit’s unconditioned nictitating membrane response. Behavioral Neuroscience. 1995;109:24–33. doi: 10.1037//0735-7044.109.1.24. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Shi T, Pineda SI, Buck DL. Conditioning the unconditioned response: modification of the rabbit’s (Oryctolagus cuniculus) unconditioned nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:144–156. doi: 10.1037//0097-7403.26.2.144. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Burhans LB. Classical conditioning and conditioning-specific reflex modification of rabbit heart rate as a function of unconditioned stimulus location. Behavioral Neuroscience. 2011a;125:604–612. doi: 10.1037/a0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Burhans LB. Incubation of conditioning-specific reflex modification: implications for post traumatic stress disorder. Journal of Psychiatric Research. 2011b;45:1535–1541. doi: 10.1016/j.jpsychires.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Burhans LB. Unpaired extinction: implications for treating post-traumatic stress disorder. Journal of Psychiatric Research. 2011c;45:638–649. doi: 10.1016/j.jpsychires.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Smith-Bell CA, Darwish DS, Stankovic G, Sparks DL. High dietary cholesterol facilitates classical conditioning of the rabbit’s nictitating membrane response. Nutritional Neuroscience. 2007;10:31–43. doi: 10.1080/10284150701232034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: US intensity effects. Learning & Behavior. 2003;31:292–298. doi: 10.3758/bf03195990. [DOI] [PubMed] [Google Scholar]
- Searcy CP, Bodabilla L, Gordon WA, Jacques S, Elliott L. Pharmacological prevention of combat-related PTSD: a literature review. Military Medicine. 2012;177:649–654. doi: 10.7205/milmed-d-11-00390. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Brennan FX, Beck KD, Beldowicz D, Coyle-DiNorcia K. Stress facilitates acquisition of the classically conditioned eyeblink response at both long and short interstimulus intervals. Learning and Motivation. 2001;32:178–192. [Google Scholar]
- Sherry A, Henson RK. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. Journal of Personality Assessment. 2005;84:37–48. doi: 10.1207/s15327752jpa8401_09. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Annals New York Academy of Sciences. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. Hyperarousal does not depend on trauma-related contextual memory in an animal model of posttraumatic stress disorder. Physiology & Behavior. 2007;90:103–107. doi: 10.1016/j.physbeh.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. Journal of Psychiatry and Neuroscience. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitization: a tale of brain and body. Part 2: animal models. Neuroscience and Biobehavioral Reviews. 2007;31:558–584. doi: 10.1016/j.neubiorev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Tsai J, Harpaz-Rotem I, Pietrzak RH, Southwick SM. The role of coping, resilience, and social support in mediating the relation between PTSD and social functioning in veterans returning from Iraq and Afghanistan. Psychiatry. 2012;75:135–149. doi: 10.1521/psyc.2012.75.2.135. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Korhonen T. Interpositus nucleus inactivation reduces unconditioned response amplitude after paired but not explicitly unpaired treatment in rabbit eyeblink conditioning. Neuroscience Letters. 2001;308:181–184. doi: 10.1016/s0304-3940(01)02000-6. [DOI] [PubMed] [Google Scholar]
- Wrenn GL, Wingo AP, Moore R, Pelletier T, Gutman AR, Bradley B, et al. The effect of resilience on posttraumatic stress disorder in trauma-exposed inner-city primary care patients. Journal of the National Medical Association. 2011;103:560–566. doi: 10.1016/s0027-9684(15)30381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Flory JD. Differentiating biological correlates of risk, PTSD, and resilience following trauma exposure. Journal of Traumatic Stress. 2007;20:435–447. doi: 10.1002/jts.20260. [DOI] [PubMed] [Google Scholar]
- Zohar J, Juven-Wetzler A, Sonnino R, Cwikel-Hamzany S, Balaban E, Cohen H. New insights into secondary prevention in post-traumatic stress disorder. Dialogues in Clinical Neuroscience. 2012;13:301–309. doi: 10.31887/DCNS.2011.13.2/jzohar. [DOI] [PMC free article] [PubMed] [Google Scholar]