Abstract
Spironolactone is a drug derived from sterols that exhibits an incomplete oral absorption due to its low water solubility and slow dissolution rate. In this study, formulations of spironolactone with four disintegrants named as croscarmellose sodium, crospovidone, sodium starch glycolate and microcrystalline cellulose II (MCCII) were conducted. The effect of those disintegrants on the tensile strength, disintegration time and dissolution rate of spironolactone-based compacts was evaluated using a factorial design with three categorical factors (filler, lubricant, and disintegrant). The swelling values, water uptake and water sorption studies of these disintegrants all suggested that MCCII compacts disintegrate by a wicking mechanism similar to that of crospovidone, whereas a swelling mechanism was dominant for sodium starch glycolate and croscarmellose sodium. The disintegration time of MCCII and sodium starch glycolate remained unchanged with magnesium stearate. However, this lubricant delayed the disintegration time of crospovidone and croscarmellose sodium. MCCII presented the fastest disintegration time independent of the medium and lubricant employed. The water sorption ratio and swelling values determined sodium starch glycolate followed by croscarmellose sodium as the largest swelling materials, whereas crospovidone and MCCII where the least swelling disintegrants. The swelling property of sodium starch glycolate and croscarmellose sodium was strongly affected by the medium pH. The disintegration time of spironolactone compacts was faster when starch was used as a filler due to the formation of soft compacts. In this case, the type of filler employed rather than the disintegrant had a major effect on the disintegration and dissolution times of spironolactone.
KEY WORDS: disintegrant, dissolution studies, factorial experimental design, spironolactone
INTRODUCTION
Spironolactone is a diuretic steroidal drug used to treat several diseases such as edema and cirrhosis (1). It shows a poor bioavailability due to its low water solubility (28 μg/mL at 25°C) and limited dissolution rate which could affect its therapeutic effect (2). For this reason, it is critical to select the appropriate combination of a filler, disintegrant, and lubricant during the drug development stage. Immediate release drugs from compacts should be preceded by a fast compact disintegration in the gastric fluid so that the therapeutic effect takes place promptly (3). It is widely accepted that a fast compact disintegration is desirable to increase the particle surface area and hence enhance drug release, especially for poorly water-soluble APIs. Other reported factors that could affect compact disintegration time are particle size, granules shape, and compact porosity (4).
Currently, there are a great number of materials called disintegrants, which are made by modification of natural polymers or by chemical synthesis (5). Recently, a new generation of disintegrants called superdisintegrants emerged. These trigger compact disintegration within few minutes upon contact with water. Sodium starch glycolate, crospovidone and croscarmellose sodium are examples of the most commonly used superdisintegrants (6). They increase the hydrostatic pressure acting either via swelling or by water wicking, or by combination of these mechanisms (7,8).
Recently, a new cellulose II-based excipient was introduced as a potential filler-binder. It is denser, but less crystalline and ductile than microcrystalline cellulose I (MCCI). Further, microcrystalline cellulose II (MCCII) shows a lower degree of polymerization and faster compact disintegration than MCCI products independent of the processing conditions (9–11). However, there are no reported studies that evaluate the suitability of MCCII as disintegrant.
The aim of this work is to evaluate the disintegrating ability of MCCII in comparison to conventional superdisintegrants such as Polyplasdone® XL-10, Explosol® and croscarmellose sodium in distilled water and 0.1 N HCl and to use a full factorial design to assess their functionality in spironolactone compacts.
MATERIALS AND METHODS
Materials
Concentrated hydrochloric acid (lot 2612KLHV) and magnesium stearate (lot 2256KXDS) were obtained from Mallinckrodt Specialty Chemicals Co (St. Louis, MO), sodium lauryl sulfate (lot 98488) was obtained from Fisher Scientific (Fair Lawn, NJ), sodium starch glycolate (Explosol®, lot 19034/08) was purchased from Blanver (Sao Paulo, Brazil), crospovidone (Polyplasdone® XL-10, lot S10906) was obtained from International Specialty Products (Wayne, NJ), croscarmellose sodium (lot T353NDR63) and Avicel® PH-101 (lot 2339) were purchased from FMC Biopolymers (Philadelphia, PA), Starch®1500 (lot IN504089) was obtained from Colorcon (West Point, PA), lactose monohydrate (lot 8596021361) was received from Foremost Farms (Baraboo, WI), talc (lot M032) was purchased from Protokimica (Medellin, Colombia), and spironolactone (lot MP1122) was donated from Humax Pharmaceuticals (Medellin, Colombia).
Methods
Preparation of Microcrystalline Cellulose II
Approximately, 150 g of wood cotton was added to a round bottom flask containing 1,800 mL of 2 N HCl. The acid hydrolysis was then conducted for 2 h at 105°C in a heating mantle (EM0500/CMK4, Electrothermal, USA). The residue thus obtained was then washed with distilled water until reaching a pH from 5 to 7. This product was soaked for 72 h in a 7.5 N sodium hydroxide solution at room temperature. After 72 h, the resulting cake was filtered and washed with distilled water until reaching a pH from 5 to 7. An amount of slurry equivalent to ∼50 g of dry sample was sequentially passed through a 3,350, 2,000, 711, 425, and 150 μm screen sizes using an oscillating granulator (Riddhi Pharma Machinery, Gulabnagar, India) at moisture contents of ∼60%, 50%, 40%, 30%, and 20%, respectively. The final powder was dried on a fluid bed drier (Leflu, Indemec, Medellin, Colombia) for 15 min at 50°C.
Experimental Design
A full factorial design composed of 24 runs and three categorical factors including a binder (i.e., lactose, Avicel® PH-101 or Starch® 1500), lubricant (i.e., magnesium stearate or talc) and disintegrant (i.e., Polyplasdone® XL-10, croscarmellose sodium, Explosol® and MCCII) was employed. The dissolution time (t80) of spironolactone, compact disintegration time and compact tensile strength were treated as responses. The Design-Expert software (version 8.0, StatEase, Inc, Minneapolis, MN) was used for the statistical analysis.
Powder Properties
Moisture content was determined by gravimetry on a convection oven (U50, Memmert, Schwabach, Germany). MCCII and Polyplasdone® XL-10 were dried at 105°C for 3 h, Explosol® at 130°C for 90 min, and croscarmellose sodium at 105°C for 6 h according to the USP32/NF29 specifications. Powder porosity (ε) was determined from the equation: 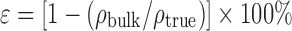 , where ρbulk and ρtrue correspond to the bulk and true densities, respectively. True density was obtained on a helium pycnometer (Accupyc II 1340, Micrometrics, USA) with ∼4 g of sample. Optical micropictures were obtained on an optical microscope (MM-180, Boeco, Germany) coupled with a digital camera (DCR-SX45, Sony, Tokyo, Japan) at a ×700 magnification.
, where ρbulk and ρtrue correspond to the bulk and true densities, respectively. True density was obtained on a helium pycnometer (Accupyc II 1340, Micrometrics, USA) with ∼4 g of sample. Optical micropictures were obtained on an optical microscope (MM-180, Boeco, Germany) coupled with a digital camera (DCR-SX45, Sony, Tokyo, Japan) at a ×700 magnification.
Swelling Value and Water Uptake Analyses
The swelling value was expressed as the ratio of the powder expanded volume upon water sorption and the initial sample weight as reported previously by Edge et al. (12). Approximately 500 mg of powder was vigorously dispersed in a graduate cylinder filled with 10 mL of simulated gastric fluid (0.1 N HCl, pH = 1) and simulated intestinal fluid (phosphate buffer, pH = 6.8) at room temperature. The cylinder was placed on a flat surface and the increase in volume was measured with time. The swelling value at each time point was then calculated by dividing the sediment volume by the sample weight.
Water uptake was determined following the procedure of Zhao and Augsburger, with minor modifications (13). It measures the water sorption of a material upon addition of water. Briefly, a funnel (6 cm diameter), attached to a Tygon tubing, was placed on an analytical microbalance (Vibra, Shinko Denshi Co., Tokyo, Japan) held on a tripod stand. The Tygon tubing delivered water into a collecting vessel placed next to the balance. A Whatman filter paper (90 mm diameter) was wetted with aqueous medium and placed in the funnel. An accurately weighted amount of the test material (∼500 mg) was then added. Ten milliliters (10 mL) of aqueous medium was then poured into the funnel through the filter paper. The change in weight as a function of time was then recorded. The measurement was stopped when a stable weight reading was observed. The difference of weights, with and without powder, as a function of time, was taken as the water uptake ability of the powder.
Preparation of Compacts
Blends of approximately 15 g of the components shown in Table I were made on a V-blender for 20 min at 15 rpm (Riddhi Pharma Machinery, RiddhiState, Gulabnagar, India). Each blend was then passed through a size 80 screen. Compacts of ∼500 mg were composed of a filler-binder (74%), spironolactone (20%), disintegrant (5%), and lubricant (1%), respectively. Compacts were manufactured on a single punch tablet machine (Compac 060804, Indemec, Itagüí, Columbia) equipped with a 13-mm flat-faced punches and die tooling at a compression pressure of 110 MPa and a dwelling time of 1 s. The upper punch was equipped with a load cell (LCGD-10K, Omega Engineering, Inc, Stanford, CT) and a strain gauge meter (DPiS8-EI, Omega Engineering, Inc, Stanford, CT). Compact porosities were determined by the expression 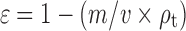 . Where ε, m, v, and ρt correspond to the porosity, mass, volume, and the true density of the powder mixture found on a helium pycnometer (AccupycII 1340, Micromeritics, Norcross, GA, USA), respectively. The resulting compact porosities remained between 0.15 and 0.2.
. Where ε, m, v, and ρt correspond to the porosity, mass, volume, and the true density of the powder mixture found on a helium pycnometer (AccupycII 1340, Micromeritics, Norcross, GA, USA), respectively. The resulting compact porosities remained between 0.15 and 0.2.
Table I.
Experimental Matrix for Formulations Containing Disintegrants
| Lot | Filler | Lubricant | Disintegrant | t 80 (min) | DT (s) | TS (MPa) |
|---|---|---|---|---|---|---|
| n = 3 | n = 3 | n = 3 | ||||
| 1 | L | MS | E | 30.2 ± 2.3 | 22.3 ± 2.5 | 2.1 ± 0.0 |
| 2 | S | T | MCCII | 14.1 ± 1.3 | 69.0 ± 4.0 | 2.0 ± 0.1 |
| 3 | M | T | E | 25.9 ± 1.6 | 37.0 ± 5.0 | 2.2 ± 0.1 |
| 4 | S | T | P | 21.1 ± 0.8 | 39.0 ± 4.0 | 2.0 ± 0.0 |
| 5 | L | MS | C | 24.9 ± 0.5 | 11.3 ± 1.5 | 2.1 ± 0.0 |
| 6 | S | MS | C | 4.3 ± 0.2 | 50.3 ± 6.1 | 2.1 ± 0.1 |
| 7 | L | MS | MCCII | 24.5 ± 1.3 | 374.7 ± 37.1 | 2.2 ± 0.0 |
| 8 | M | MS | P | 23.1 ± 1.1 | 30.3 ± 4.7 | 2.1 ± 0.0 |
| 9 | M | MS | MCCII | 34.5 ± 2.1 | 152.7 ± 30.0 | 2.1 ± 0.0 |
| 10 | M | T | P | 24.8 ± 1.3 | 15.3 ± 2.5 | 2.1 ± 0.0 |
| 11 | M | MS | C | 21.8 ± 0.4 | 13.3 ± 1.5 | 2.1 ± 0.0 |
| 12 | S | T | C | 15.6 ± 0.1 | 54.0 ± 7.0 | 2.1 ± 0.0 |
| 13 | L | T | P | 16.8 ± 0.3 | 19.7 ± 4.0 | 2.1 ± 0.1 |
| 14 | S | MS | E | 12.1 ± 0.1 | 70.0 ± 5.6 | 2.0 ± 0.1 |
| 15 | L | T | MCCII | 26.6 ± 1.7 | 74.7 ± 11.2 | 2.1 ± 0.0 |
| 16 | S | MS | MCCII | 32.8 ± 1.8 | 53.0 ± 4.6 | 2.0 ± 0.0 |
| 17 | M | T | MCCII | 41.4 ± 2.4 | 1379.3 ± 232.0 | 2.2 ± 0.0 |
| 18 | M | MS | E | 22.3 ± 1.3 | 14.7 ± 2.5 | 2.1 ± 0.0 |
| 19 | L | T | E | 25.3 ± 1.3 | 18.7 ± 4.0 | 2.1 ± 0.0 |
| 20 | L | T | C | 22.8 ± 1.1 | 11.3 ± 1.5 | 2.1 ± 0.0 |
| 21 | S | MS | P | 14.4 ± 0.2 | 51.7 ± 6.5 | 2.0 ± 0.0 |
| 22 | L | MS | P | 19.8 ± 0.3 | 19.7 ± 1.5 | 2.1 ± 0.0 |
| 23 | M | T | C | 20.7 ± 0.4 | 25.0 ± 3.0 | 2.1 ± 0.0 |
| 24 | S | T | E | 11.4 ± 0.1 | 38.3 ± 6.5 | 2.1 ± 0.1 |
| VR | S | MS | C | 6.4 ± 0.9 | 52.3 ± 7.3 | 2.1 ± 0.1 |
Data are expressed as mean ± SD in triplicate; p < 0.05
L lactose; S starch; M microcrystalline cellulose (Avicel® PH-102); T talc; MS magnesium stearate; E Explosol®; C Croscarmellose sodium®; P Polyplasdone® XL-10; MCCII microcrystalline cellulose II; DT disintegration time; t 80 time to release 80% of spironolactone; TS compact tensile strength; VR validation run with the optimum composition according to the factorial model: S(74%), MS(1%), C(5%) and spironolactone (20%)
Dissolution Studies
The concentration of spironolactone in each mixture was found by UV analysis (HACH DR500, HACXH Company, Loveland, CO) at 242 nm and found to be within 95–105% according to the USP32/NF29 specifications. A calibration curve was built at concentrations of 5, 10, 15, 20, and 25 μg/mL. Dissolution studies were conducted employing an Erweka (DT6-K, Erweka GmbH, Milford, CT) type 2 apparatus containing 1,000 mL of 0.1 N HCl and 0.1% sodium lauryl sulfate at 37°C and 75 rpm for 1 h. At 5, 10, 15, 30, 45, and 60 min, aliquots of 1 mL each, were taken, filtrated, and diluted (200 μL/1,000 μL) before measurement. A UV–Vis spectrophotometer (HACH DR500, HACXH Company, Loveland, CO) was used for the analysis at 242 nm. The Weibull model with two parameters was employed to determine the t80 (14).
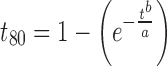 |
1 |
Where a and b correspond to the dissolution rate and the dissolution type (Fickian or non-Fickian), respectively. The Statgraphic software (version 4.0, StatPoint, Inc, USA) was used for the non-linear fitting employing the least squares method.
Disintegration Studies
A disintegrator (39-133-115, Hanson Research Corporation, Northridge, CA) without discs was employed. The test was conducted in simulated gastric and intestinal fluids at 37 ± 2°C and at 30 strokes/min (15).
Compact Tensile Strength and Porosity
Compact crushing strength was determined on a Hardness tester (Model 24041869, Stokes, USA). Compact tensile strength (TS) was obtained from the crushing strength values using the Fell and Newton equation: (16)
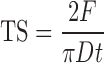 |
2 |
Where, F is the crushing strength needed to break the compact into two halves, D is the diameter of the compact (in millimeters), and t is the compact thickness (in millimeters).
Compact Water Sorption Ratio
The method of Bi et al. was employed (17). This method evaluates the ability of the compact to uptake water. A compact was placed on a petri dish containing a Kimwipe paper with 6 mL of water. Once the tablet was completely wet, the water sorption ratio (WSR) was calculated according to the relationship: WSR = (Wa − Wb)/Wb, where Wb and Wa are the compact weights before and after water sorption, respectively.
RESULTS
Figures 1 and 2 show the swelling values and the water uptake capacity of MCCII and commercial superdisintegrants. Highly swelling materials such as Explosol® and croscarmellose sodium had the largest water uptake ability. However, less soluble materials or capillary-driven materials such as MCCII had very low water uptake values. The degree of swelling upon water uptake is depicted in Fig. 3.
Fig. 1.
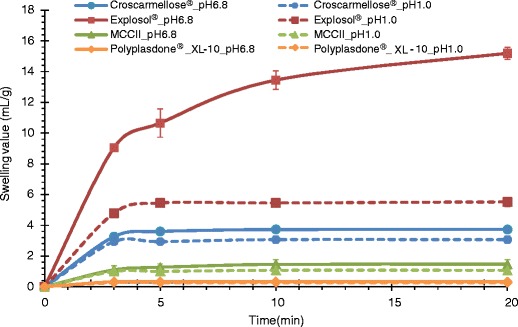
Swelling values of MCCII and commercial superdisintegrants in simulated gastric and intestinal fluids
Fig. 2.
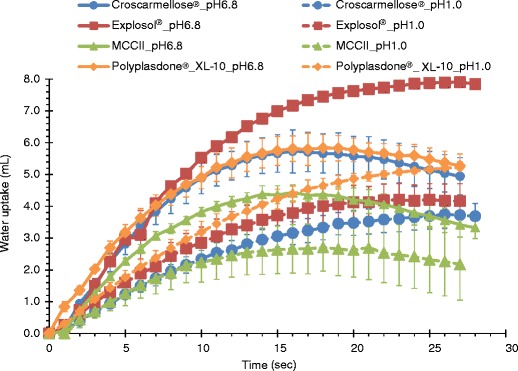
Water uptake ability of MCCII and commercial superdisintegrants in simulated gastric and intestinal fluids
Fig. 3.
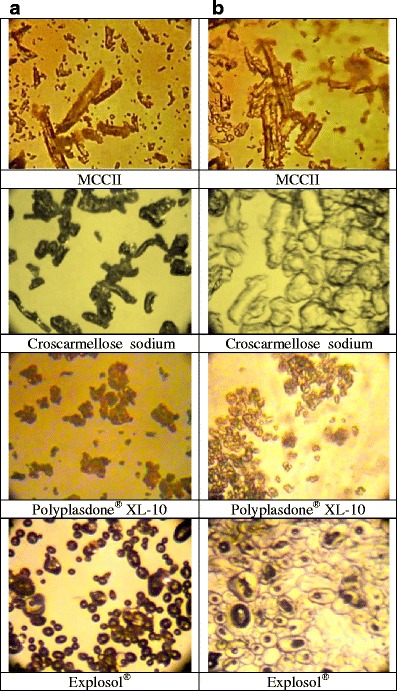
Morphology of disintegrant particles: a before and b 5 s after addition of simulated intestinal fluid at ×700 magnification
In general, the ability of these materials to swell decreased when placed in the acid media. This finding was more drastic for highly swelling materials such as Explosol® and croscarmellose sodium. On the contrary, the swelling ability of Polyplasdone® XL-10 was virtually independent to the medium pH. In fact, less swelling materials such as MCCII and Polyplasdone® XL-10 had a minor swelling reduction in acid medium. Therefore, independent of the medium pH, the swelling ability can be ranked as Explosol® > croscarmellose sodium > MCCII > Polyplasdone® XL-10.
Sodium starch glycolate, croscarmellose sodium, and MCCII took about 15–25 s to attain a maximum uptake (Fig. 2). Beyond these times, unretained excess water by the powder drained off. Alternately, most swelling materials retained a larger amount of water. The amount of water uptake was more prominent for highly swelling materials, whereas capillary-driven materials exhibited lower values.
The water uptake rates can be obtained from the slopes of the water uptake curves within the first 10 s upon water sorption. The acid media reduced the water uptake ability of the disintegrants. For this reason, in distilled water Explosol®, croscarmellose sodium, Polyplasdone® XL-10, and MCCII had large water uptakes in the pH 6.8 medium (0.60, 0.55, 0.52, and 0.43 g/s, respectively), but in acid media (pH 1.0) Explosol® and Polyplasdone® XL-10 had comparable, but reduced water uptakes (0.30 and 0.32 g/s, respectively) and croscarmellose sodium and MCCII presented even lower rates (0.26 g/s 0.25 g/s, respectively). For this reason, these rates were high in swelling materials and low for water wicking materials such as Polyplasdone® XL-10 and MCCII. These rates in acid media were essentially lower (0.25–0.33 g/s).
Figure 4 shows the water sorption ratio of compacts made with the disintegrants after soaking them in 10 mL of both media. In this case, MCCII, croscarmellose sodium, and Polyplasdone® XL-10 showed the lowest values, whereas sodium starch glycolate showed the highest value. In general, the water sorption ratio did not vary between the different media. These results are opposed to those exhibited by the powders. On the other hand, highly swelling materials such as Explosol® presented the largest water sorption ratio due to their high ability to draw and retain water leading to a size increase. Conversely, MCCII and Polyplasdone® XL-10 had a high capillary action and a low swelling. The rank order of water sorption ratio was: sodium starch glycolate > croscarmellose sodium > Polyplasdone® XL10 > MCCII.
Fig. 4.
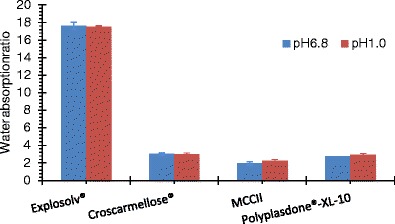
Water sorption ratio of MCCII and commercial superdisintegrants in simulated gastric and intestinal fluids
Figure 5 shows the effect of the media pH and lubricant (magnesium stearate) on the compact disintegration of these materials. Acid media (i.e., 0.1 N HCl) and buffer phosphate pH 6.8 were selected to simulate gastric and intestinal conditions. Independent of the medium pH and lubricant employed, MCCII compacts presented the fastest disintegration and Polyplasdone® XL-10 the slowest values due to the formation of strong compacts.
Fig. 5.
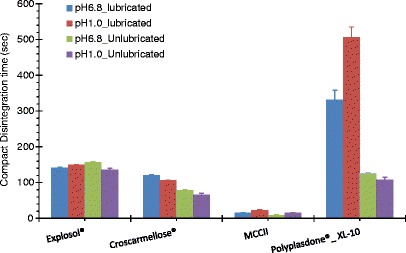
Compact disintegration times of MCCII and commercial superdisintegrants in simulated gastric and intestinal fluids, with and without magnesium stearate
In general, compacts containing talc had slightly faster disintegration and dissolution times than those containing magnesium stearate due to the hydrophobic character of magnesium stearate which delays water penetration into the compact. Formulation 17 presented the largest disintegration time (∼1,379 s), followed by formulation 7 (∼375 s). As a consequence, the use of MCCII as disintegrant in combination with Avicel® PH-101 and lactose due to the formation of strong compacts, delayed compact disintegration and spironolactone dissolution (41.4 vs. 24.5 min, respectively). In this case, it is advisable to employ highly swelling disintegrants such as sodium starch glycolate and croscarmellose sodium (Table I).
Figure 6a depicts the factorial model for disintegration time with respect to disintegrant and filler utilized in a formulation. Independent of the lubricant used, disintegration times were very low, except when MCCII was used as disintegrant in combination with microcrystalline cellulose I (Avicel® PH-101) or lactose. On the other hand, if starch is used as diluent, disintegration times were lower than 65 s, mainly due to the hydrophilic nature of starch and the formation of relatively soft compacts.
Fig. 6.
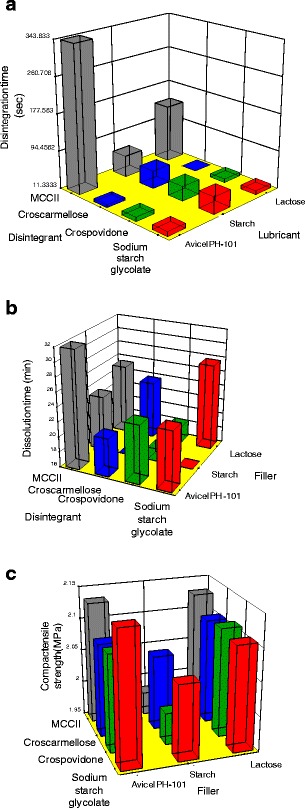
Effect of filler and disintegrant on a disintegration time, b dissolution time and c compact tensile strength at a constant lubricant level according to the factorial models
Figure 6b depicts the results of the factorial model for the dissolution time of spironolactone (t80) as a function of filler and disintegrant. The dissolution time when Avicel® PH-101 and lactose were used as fillers was less than 27 min, except when MCCII was present in the formulation (∼32 min). Further, with starch, low dissolution times were obtained (<18 min), except in combination with MCCII (∼23 min). Further, when a highly binding material is used as a filler (Avicel® PH-101) in combination with commercial disintegrants, dissolution times ranged from 20 to 22 min. If lactose is used as filler, disintegration times were about 12 s except in combination with MCCII due to its contribution on compact strength as discussed previously. This effect was also seen for Avicel® PH-101 due to the formation of stronger compacts than lactose. The effect of diluent on disintegration time (from the fastest to the slowest) varied as lactose ≅ Avicel® PH-101 > starch when formulated with commercial disintegrants. Likewise, formulations containing MCCII as disintegrant always rendered the slowest disintegration time, especially in combination with Avicel® PH-101. This indicates that both diluent and disintegrant affected compact disintegration.
Figure 6c depicts the variation of the compact tensile strength with respect to disintegrant and filler composition. The tensile strength of compacts having lactose and Avicel® PH-101 was comparable and larger than those produced from starch compacts. In fact, the low tensile strength of compacts containing starch were inversely correlated with the fast spironolactone release shown by compacts which contained starch as diluent. In other words, the formation of soft compacts also favored a fast dissolution.
The complete factorial model coefficients for the three responses are shown as follows:
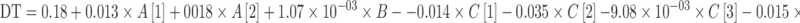 |
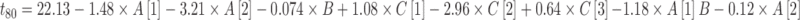 |
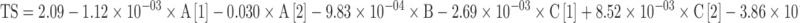 |
Where A, B, C, DT, t80, and TS correspond to the filler, lubricant and disintegrant types, disintegration time, time to release 80% of spironolactone and compact tensile strength, respectively.
DISCUSSION
A swelling value describes the ability of the powder to increase in size/volume due to water uptake by their particles. Sodium starch glycolate showed the highest swelling with croscarmellose sodium being next. In comparison, MCCII and Polyplasdone® XL-10 showed substantially less swelling. The time to peak is interpreted as the time needed to reach a maximum water saturation of the particle pores while absorbing water. In this case, the largest values were observed for highly swelling materials, especially Explosol® and the lowest values were observed for capillary-driven materials. In the first case, water is absorbed in the particle pores to the interior and in this process water migration causes the polymer chains to expand creating more space among them. As water is migrating between the chains, a three-dimensional network is created in which water is retained. The time to peak is reached once there is a sufficient number of polymer chains with inter-and intramolecular hydrogen bonds available to bind liquid water.
On the contrary, for capillary-driven materials such as MCCII, water uptake begins through the particle pores followed by migration into the interior of the capillaries. In this case, particle expansion is negligible since capillaries and particles experienced a minor disruption attributable to particle swelling. The time to peak (∼10 s) in these materials was shorter than that showed by swelling materials (15–25 s) since the former did not form a viscous halo upon water uptake. It has been reported that the larger sodium starch glycolate swelling compared to croscarmellose sodium is due to its ability to carry out a three-dimensional swelling, whereas, croscarmellose sodium is only able to swell in two dimensions causing almost no change in the fiber length (18). In fact, Fig. 3 depicts a large three-dimensional swelling of the tiny semispherical Explosol® particles. Likewise, the elongated croscarmellose sodium particles also showed a partial three-dimensional volume expansion. On the other hand, Polyplasdone® XL-10 exhibited irregular and porous particles with a rough surface and upon water uptake formed particle aggregates of larger sizes. Further, MCCII showed elongated particles with virtually no volume expansion upon water uptake.
The fast water uptake and high swelling ability of sodium starch glycolate and croscarmellose sodium is attributed to the presence of a carboxylic moiety and their ability to quickly hydrate and produce gels upon water uptake. The slightly higher water uptake rate of sodium starch glycolate compared to croscarmellose sodium could be ascribed to the smaller particle size and higher amorphous content which eased water accessibility (19).
The large water uptake rate of Polyplasdone® XL-10 is probably due to its own cross-linked nature causing entanglement of the polymer chains making it water insoluble with the ability to take up water quickly without considerable swelling (20).
A low crystallinity is not the only requirement for a rapid water uptake since sodium starch glycolate and croscarmellose sodium had low (12%) and high crystallinity (45%), respectively and exhibited approximately the same uptake rates (0.60 vs. 0.55 mL/s). For this reason, the difference observed in water uptake was mainly attributed to the disintegrants chemical nature, rather than their physical properties such as particle size, morphology, porosity and crystallinity. For instance, Explosol® is the sodium salt of a carboxymethyl ether of starch. It is produced by crosslinking starch with sodium trimethylphosphate followed by carboxymethylation with sodium monochloroacetate. In this case, a phosphate group, which links the polymer chains allows for more spacing and water accessibility to form hydrogen bonds with incoming water molecules. On the contrary, croscarmellose sodium is a cross-linked polymer of carboxymethyl cellulose sodium and sodium glycolate. Since this is a linear cellulosic derivative, the polymer chains are closer limiting water accessibility and hydrogen bonding with hydroxyl groups and hydration of the carboxyl moieties.
The water sorption ratio of compacts was determined on compacts resting on a wet surface. For this reason, compact water migration occurred from its base to the top causing axial expansion due to water disruption of the particles and a minor radial growth. Highly swelling materials formed the largest water sorption ratios. Polyplasdone® XL-10 expanded forming a highly porous sponge-like structure as a result of water interaction and disruption of its polymeric chains. Further, it has been reported a high porosity (∼80%) for this material providing void spaces for a substantial water penetration inside the compact matrix (19). Conversely, compacts of MCCII cracked when put in contact with water and showed minimal water sorption ratios.
Compact disintegration in capillary and swelling materials occurs due to the rapid replacement of intermolecular hydrogen bonding and disruption of van der Waals forces of the particles with incoming water molecules. The high compact swelling produced upon contact with water did not cause a delay in disintegration time despite of the three-dimensional gel produced upon contact in the aqueous medium. On the other hand, croscarmellose sodium had a moderate disintegration time due to its lower swelling ability compared to that of Explosol®.
For water wicking materials, there was no correlation between interparticle bonding strength and disintegration time. For instance, a material can form compacts of high strength showing a low disintegration time as observed for Polyplasdone® XL-10. However, for sodium starch glycolate, which was the best swelling material, disintegration time was moderate due to the formation of a gel layer which delays disintegration. Croscarmellose sodium had particles with a moderate swelling rendering soft compacts with a fair disintegration time.
Considering the water uptake rate and water sorption ratio of sodium starch glycolate and croscarmellose sodium, it appears that the faster compact disintegration observed for croscarmellose sodium can be attributed to its moderate swelling propensity and the formation of weak compacts. As seen previously, sodium starch glycolate and croscarmellose sodium compacts disintegrate via swelling. On the other hand, MCCII showed a fast water wicking and compact bursting. In the case of Polyplasdone® XL-10, it has been reported that its fast disintegration is due to the combined effect of the high powder porosity and irregular particle morphology that leads to a quick water wicking action and compact disintegration (21).
Compact disintegration times of MCCII and Explosol® were virtually not affected by the presence of lubricant and medium pH. On the other hand, independent of the media pH, the presence of magnesium stearate lingered the disintegration time of croscarmellose sodium and Polyplasdone® XL-10 (Fig. 5). Further, Polyplasdone® XL-10 and croscarmellose sodium were the only disintegrants that showed a significant disintegration time difference in both media. This difference could be ascribed to different sorption abilities in the two media due to the uncoiling of the polymer chain in the acid media. On the other hand, the swelling capacity decrease shown by croscarmellose sodium is attributed to the conversion of the carboxymethyl sodium salt moiety to its free acid form in acidic media. This acid form is less hydrating and swelling than its salt form, and hence, the water holding capacity is reduced in acid medium (13). Opposed to the powder, this effect was not observed for sodium starch glycolate compacts, which also have a carboxymethyl sodium moiety. This might be due to the linear nature of the cellulose polymer chains and its high crystallinity which limits water accessibility to these moieties when particles are consolidated into a compact.
For sodium starch glycolate, the highly swelling ability of the compacts is due to the amylopectin component of starch, which is also responsible for its high water retention (22). It has been reported that this material is not affected by the medium pH in a range from 3 to 7. However, at pHs >7, viscosity increases due to the reorientation or random coiling of the amylopectin component forming a viscous layer in contact with water delaying further water penetration (23). On the contrary, no change in disintegration time was found for MCCII due to the absence of the carboxylic moiety.
Table I shows the composition of the spironolactone-based formulations and the resulting disintegration and dissolution times, and compact tensile strength, respectively. In general, a fast dissolution time (t80) of <16 min was triggered by the synergistic effect of the high swelling action of croscarmellose sodium or Explosol® in combination with starch leading to a rapid compact disintegration. For this reason, the hydrophilicity of starch particles causes water wicking through their pores accelerating compact disintegration. Therefore, a formulation containing starch promotes the rapid dissolution of spironolactone. The large water wicking ability of MCCII is attributed to capillary formation and disruption of hydrogen bonds. However, MCCII did not favor compact disintegration due to its limited swelling ability which is crucial when dealing with strong compacts. Since spironolactone is a poorly soluble drug, a rapid compact disintegration is important for a fast contact of the drug with the dissolution medium. In all cases, disintegration times were lower than dissolution times, and thus the combined effect of a filler and disintegrant played a major role in drug dissolution. Moreover, croscarmellose sodium in combination with lactose rendered compacts with a rapid drug dissolution. This indicates that for less water-soluble diluents such as microcrystalline cellulose and starch, water wicking disintegrants such as crospovidone were also effective. On the other hand, for water-soluble diluents such as lactose, a moderate swelling material such as croscarmellose sodium was more desirable.
Figure 3 depicts Polyplasdone® XL10 particles with rough surfaces, which upon water uptake forms aggregates of larger size. However, this process does not have any effect on spironolactone release since it was always rapid when Polyplasdone® XL10 was present (∼14–25 s). This indicates that the aggregate state eases water penetration and does not retain this drug within the particles. In fact, the high proportion of filler eased the formation of soft compacts and was more determined than the presence of disintegrant for the rapid drug release.
Table II lists the ANOVA results of the linear models for disintegration time, dissolution time, and tensile strength of spironolactone compacts. The effect of filler and disintegrant was considered as significant, whereas the effect of lubricant in the formulation was not important. Moreover, the ANOVA table for dissolution time of spironolactone shows that only the effect of the filler in the formulation was considered as significant. This implies that the contribution of lubricant and disintegrant was negligible. Further, the ANOVA results for compact tensile strength also shows that only the filler effect was considered as significant. A high coefficient of determination implies a good fit to the linear model. In other words, 82.8% to 95.3% of the variability can be attributed to the model and 4.7% to 17.2% can be attributed to random error.
Table II.
ANOVA Table of the Models for Disintegration, Dissolution and Tensile Strength of Spironolactone Compacts
| Model | Source of error | SS | df | MS | F value | p value (α = 0.05) |
|---|---|---|---|---|---|---|
| Disintegration time (s) | Model | 0.12 | 17 | 6.87 × 10−03 | 4.88 | 0.030 |
| A (filler) | 0.019 | 2 | 9.27 × 10−03 | 6.59 | 0.031 | |
| B (lubricant) | 2.75 × 10−05 | 1 | 2.75 × 10−05 | 0.02 | 0.893 | |
| C (disintegrant) | 0.064 | 3 | 0.021 | 15.11 | 0.003 | |
| AB | 3.91 × 10−03 | 2 | 1.95 × 10−03 | 1.39 | 0.320 | |
| AC | 0.028 | 6 | 4.62 × 10−03 | 3.28 | 0.087 | |
| BC | 2.72 × 10−03 | 3 | 9.05 × 10−04 | 0.64 | 0.615 | |
| Residual | 8.45 × 10−03 | 6 | 1.41 × 10−03 | |||
| Corrected total | 0.13 | 23 | ||||
| Standard deviation | 0.038 | r 2 | 0.9325 | |||
| Dissolution time (min) | Model | 1250.53 | 17 | 73.56 | 1.69 | 0.266 |
| A (filler) | 528.63 | 2 | 264.31 | 6.09 | 0.036 | |
| B (lubricant) | 0.13 | 1 | 0.13 | 3.05 × 10−03 | 0.958 | |
| C (disintegrant) | 400.48 | 3 | 133.49 | 3.07 | 0.112 | |
| AB | 23.07 | 2 | 11.54 | 0.27 | 0.775 | |
| AC | 266.1 | 6 | 44.35 | 1.02 | 0.490 | |
| BC | 32.11 | 3 | 10.7 | 0.25 | 0.861 | |
| Residual | 260.51 | 6 | 43.42 | |||
| Corrected total | 1511.04 | 23 | ||||
| Standard deviation | 6.59 | r 2 | 0.8276 | |||
| Tensile strength (MPa) | Model | 0.063 | 17 | 3.71 × 10−03 | 7.22 | 0.011 |
| A (filler) | 0.042 | 2 | 0.021 | 41.22 | 0.000 | |
| B (lubricant) | 2.32 × 10−05 | 1 | 2.32 × 10−05 | 0.045 | 0.839 | |
| C (disintegrant) | 4.40 × 10−03 | 3 | 1.47 × 10−03 | 2.85 | 0.127 | |
| AB | 3.81 × 10−03 | 2 | 1.91 × 10−03 | 3.71 | 0.090 | |
| AC | 0.012 | 6 | 1.95 × 10−03 | 3.78 | 0.065 | |
| BC | 7.88 × 10−04 | 3 | 2.63 × 10−04 | 0.51 | 0.690 | |
| Residual | 3.09 × 10−03 | 6 | 5.14 × 10−04 | |||
| Corrected total | 0.066 | 23 | ||||
| Standard deviation | 0.023 | r 2 | 0.9534 |
SS sum of squares; df degree of freedom; MS mean square
The best formulation which renders the fastest dissolution and disintegration times was obtained from the linear models. These models revealed the optimum compact tensile strength, disintegration, and dissolution times of 2.1 MPa, 47.3 s, and 8.9 min, respectively. These predicted values are very close to the values obtained for formulation 6 which had a compact tensile strength, disintegration, and dissolution times of 2.1 MPa, 50.3 s, and 4.3 min, respectively. This formulation contained a combination of spironolactone (20%), starch (74%), magnesium stearate (1%), and croscarmellose sodium (5%) as described in Table I. In order to test the validity of the factorial models, a new lot was produced with the optimum formulation composition as described previously. It resulted in dissolution and disintegration times and compact tensile strength of 6.4 min, 52.3 s, and 2.1 MPa, which are very close to the predicted values (8.9 min, 47.3 s, and 2.1 MPa, respectively).
CONCLUSION
MCCII compacts disintegrated by water wicking similar to that exhibited by crospovidone. Conversely, sodium starch glycolate and croscarmellose sodium showed a swelling mechanism. MCCII could also be used as disintegrant although this might not the first choice to formulate very poorly soluble drugs in combination with highly binding materials.
ACKNOWLEDGMENTS
The authors thank the staff of the University of Antioquia pilot plant for providing us with the resources needed to conduct this project.
REFERENCES
- 1.Yuancai D, WaiKiong NG, Shoucang S, Sanggu K, Reginald BH. Preparation and characterization of spironolactone nanoparticles by antisolvent precipitation. Int J Pharm. 2009;375:84–88. doi: 10.1016/j.ijpharm.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Limayem BI, Charcosset C, Sfar S, Fessi H. Preparation and characterization of spironolactone-loaded nanocapsules for pediatric use. Int J Pharm. 2006;325:124–131. doi: 10.1016/j.ijpharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Niazi S. Textbook of biopharmaceutics and clinical pharmacokinetics. New York: Appleton; 2002. [Google Scholar]
- 4.Zhao N, Augsburger LL. Functionality comparison of 3 classes of superdisintegrants in promoting aspirin tablet disintegration and dissolution. AAPS PharmSciTech. 2005;6:E634–E640. doi: 10.1208/pt060479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao N, Augsburger LL. The influence of granulation on superdisintegrant performance. Pharm Dev Technol. 2006;11:47–53. doi: 10.1080/10837450500463828. [DOI] [PubMed] [Google Scholar]
- 6.Massimo G, Catellani PL, Santi P, Bettini R, Vaona G, Bonfanti A, et al. Disintegration propensity of tablets evaluated by means of disintegrating force kinetics. Pharm Dev Technol. 2000;5:163–169. doi: 10.1081/PDT-100100531. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero C, Munoz N, Velasco M, Muñoz-Ruiz A, Jimenez Castellanos R. Disintegrating efficiency of croscarmellose sodium in a direct compression formulation. Int J Pharm. 1997;147(11).
- 8.Gohel RK, Parikh RK, Brahmbhatt BK, Shah AR. Preparation and assessment of novel coprocessed superdisintegrant consisting of crospovidone and sodium starch glycolate: a technical note. AAPS PharmSciTech. 2007;8:E63–E69. doi: 10.1208/pt0801009. [DOI] [PubMed] [Google Scholar]
- 9.Rojas J, Kumar V. Assessment of the effect of spray-drying on the powder and tableting properties of microcrystalline cellulose II. Int J Res Pharm Sci. 2011;2:276–284. [Google Scholar]
- 10.Rojas J, Lopez A, Gamboa Y, Gonzales C, Montoya F. Assessment of processing and polymorphic form effect on the powder and tableting properties of microcrystalline celluloses I and II. Chem Pharm Bull. 2011;59:603–607. doi: 10.1248/cpb.59.603. [DOI] [PubMed] [Google Scholar]
- 11.Rojas J, Kumar V. Effect of polymorphic form on the functional properties of cellulose: a comparative study. Carbohydr Polym. 2012;87:2223–2230. doi: 10.1016/j.carbpol.2011.10.052. [DOI] [Google Scholar]
- 12.Edge S, Steele DF, Staniforth JN, Chen A, Woodcock P. Powder compaction properties of sodium starch glycolate disintegrants. Drug Dev Ind Pharm. 2002;28:989–999. doi: 10.1081/DDC-120006430. [DOI] [PubMed] [Google Scholar]
- 13.Zhao N, Augsburger LL. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets. AAPS PharmSciTech. 2005;6:E120–E126. doi: 10.1208/pt060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weibull W. Dissolution of fluorocortisone from phospholipid coprecipitates. J Appl Mech. 1951;18:293–297. [Google Scholar]
- 15.Pharmacopoeial Convention and the National Formulary. “United States PharmacopoeiaThe Standard of Quality”, USP32/NF27, United States Pharmacopoeial Convention, New York; 2009.
- 16.Fell JT, Newton JM. The tensile strength of lactose tablets. J Pharm Pharmacol. 1968;20:657–758. doi: 10.1111/j.2042-7158.1968.tb09832.x. [DOI] [PubMed] [Google Scholar]
- 17.Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Drug Dev Ind Pharm. 1999;95:571–581. doi: 10.1081/DDC-100102211. [DOI] [PubMed] [Google Scholar]
- 18.Troy DV, Hauber MJ, editors. Remington, the science and practice of pharmacy. 21. Washington, D.C.: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 19.Rojas J. Assessment of co-processing of cellulose II and silicon dioxide as a platform to enhance excipient functionality. PhD thesis. 2011, The University of Iowa, Iowa City, 336.
- 20.Quadir A, Kolter K. A comparative study of current superdisintegrants. Pharm Technol. 2006;10:1–4. [Google Scholar]
- 21.Gonnissen Y, Remon JP, Vervaet C. Effect of maltodextrin and superdisintegrant in directly compressible powder mixtures prepared via co-spray drying. Eur J Pharm Biopharm. 2008;68:277–282. doi: 10.1016/j.ejpb.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Mitrevej A, Faroongsarng D, Sinchaipanid N. Compression behavior of spray dried rice starch. Int J Pharm. 1996;140:61–68. doi: 10.1016/0378-5173(96)04576-0. [DOI] [Google Scholar]
- 23.German ML, Blumenfeld AL, Guenin YV, Yuryev VP, Tolstoguzov VB. Structure formation in systems containing amylose, amylopectin, and their mixtures. Carbohydr Polym. 1992;18:27–34. doi: 10.1016/0144-8617(92)90184-R. [DOI] [Google Scholar]


