Abstract
This study evaluated the effect of inhaled BaP on female reproductive function. Rats were exposed to 50, or 75 or 100 μg BaP/m3, four hours a day for 14 days via inhalation. Plasma E2, P4, LH and FSH concentrations were determined. Ovarian BaP metabolism and aryl hydrocarbon hydrolase (AHH) activity at proestrus were determined and fertility evaluations were conducted. Ovulation rate and number of pups/litter were reduced in rats exposed to 100 μg BaP/m3 compared with other treatment and control groups. Plasma concentrations of E2, and LH were significantly reduced at proestrus in BaP-exposed versus those of controls whereas those of P4 were significantly reduced at diestrus I. The activity of AHH in ovarian and liver tissues and concentrations of BaP 7,8-diol and BaP 3,6-dione metabolites increased in an exposure concentration-dependent manner. These data suggest that exposure of rats to BaP prior to mating contributes to reduced ovarian function and fetal survival.
Keywords: Benzo(a)pyrene, Inhalation, Ovulation, Meiotic maturation, Estrogen, Progesterone, Luteinizing hormone, Follicle stimulating hormone
1. Introduction
Infertility among women of reproductive age in United States of America was estimated at 4.5 – 4.9 × 106 in the 1980s and by mid 1990s the estimate increased to 6.2 × 106 [1]. Regretfully, infertility cases have been projected to increase among the aforementioned women to about 7.7 × 106 by 2025 [2]. Some infertility cases are idiopathic in nature and may originate from exposure(s) to chemicals [3,4]. Published data reveal that women are exposed to a variety of chemicals in the occupational and domestic environments. Estimates indicate that 75% of all working women are of reproductive age, and 17% of working women are exposed to toxic chemicals in the work place [5]. The reproductive physiology of females is very delicate. Only about 400 of approximately 2 million oocytes contained in primordial follicles at birth are ovulated during the reproductive life of a woman [6]. Any environmental chemical insult on the ovary during the reproductive age of females has the potential to disrupt folliculogenesis (the ovulatory process) and lead to infertility. Because of the finite number of oocytes contained in the ovary, the destruction of follicles by toxic chemicals leads to early menopause [7,8].
Epidemiological data suggest that female smokers have delayed conception [9], premature ovarian failure that leads to an earlier onset of menopause [9–11]. Furthermore, these subjects have lower success rates when using assisted reproductive technologies [12,13] compared with their non-smoking counterparts. Compelling evidence demonstrates that exposure to main-stream [14] and side-stream [15] cigarette smoke exposures can affect ovarian function [14]. However, the toxic agents present in cigarette smoke, and their mechanisms of action responsible for the adverse effects of cigarette smoke on ovarian function have yet to be determined. Isolating the chemical(s) in tobacco smoke responsible for altered fertility and accelerated menopause is complicated by the fact that cigarette smoke is composed of approximately 4000 chemicals. These chemicals include nicotine, nitroso compounds, aromatic amines, protein pyrolysates and polycyclic aromatic hydrocarbons (PAHs; [16,17]). Only a few of these have been studied for their effects on the reproductive system [18]. Of the PAHs present in cigarette smoke, we have demonstrated that a prototypical member of the PAH family of compounds (benzo(a)pyrene [BaP]) perturbs ovarian function and embryo survival [19,20]. Apart from being present at high levels in cigarette smoke, BaP is a ubiquitous environmental pollutant which is also present in polluted air, petroleum products, charbroiled foods, contaminated water in addition to occurring as a toxic bi-product of incomplete combustion of fossil fuels [21]. Clearly, humans are continuously exposed to BaP whether they smoke or not and it is of importance to establish the specific contribution of this PAH to the failure of the ovary to function.
Benzo(a)pyrene has generated a lot of interest in view of its gonadal endocrine disrupting properties [19,22–24]. Vulnerable organs such as the gonads are exposed directly to inhaled BaP without first passage through the liver for metabolism and detoxification. Our data suggest that the ovary has the necessary enzyme system for the metabolism of BaP to reactive metabolites that can disrupt normal functions of the ovary [25]. The objective of the study was to determine the effect of BaP on the regulation of ovarian steroidogenesis by the pituitary hormones and subsequently, gametogenesis and fetal survival.
2. Materials and methods
2.1. Animals and exposure
Adult female Fisher-344 rats, approximately 10 weeks of age and weighing about 300–350g were purchased from Harlan Sprague Dawley (Indianapolis, IN). Animals were housed in pairs, in polyethylene cages and allowed to acclimatize to the animal care facilities for one week prior to initiation of studies. Rats were maintained in an environmentally controlled room with a 12h light: 12h dark cycle (lights on at 0600h), 22°C and humidity range of 50–60% and allowed ad libitum access to commercial rat chow (5001 Lab meal, Ralston Purina Co., MO, USA) and water. Acclimated rats were checked for ability to maintain regular 4-day estrous cycles by daily examinations of vaginal histologies for stages of the estrous cycle according to the method of Goldman et al. [26]. To accomplish this, approximately 150 to 200 μl of sterile physiological saline were introduced carefully into the vagina of each rat with a sterile Pasteur pipette. The tip of each pipette designated for use in this procedure was fire polished in order to prevent or minimize trauma to the vaginal epithelium. Care was also taken to prevent contact with the cervix particularly at estrus, in as much as such contact triggers pseudo pregnancy. After pulling the introduced saline in and out of the vagina twice, each final aspirate was smeared on an alcohol cleaned microscope slide, followed by a 10 minute ethanol fixation, 3 minute toluidine blue staining and rinsing with deionized water. Subsequently, a drop of glycerol was placed on the stained specimen, a cover glass applied and subjected to light microscopy (magnification = 150×) for the identification of vaginal histologies characteristic of the stages of the estrous cycle. Smears with many leukocytes, which may or may not be mixed with varying number of cornified epithelial cells were classed as being representative of diestrus II. Smears with rounded polynucleated epithelial cells, which may initially occur as wispy or stringy aggregates or numerous clumps were classed as being representative of proestrus. Those exhibiting prominence of cornified epithelial cells or dispersed non-nucleated cells with variable number of leukocytes were considered to depict estrus or diestrus 1, respectively.
Rats that exhibited approximately 4-day estrous cycles consistently for three cycles were assigned randomly to three treatment groups and a control group (N= 20/group). Before treatment, rats were acclimated to a 52 port Cannon nose-only exposure chambers for 4hr a day for 3 days. Treatments consisted of sub-acute exposure of rats to 50, 75 or 100μg BaP/m3 continuously via nose-only inhalation for 4hr on a daily basis for 14 days using a state-of-the-art dual-component aerosol generator developed in our laboratory. Carbon black (CB) was used as the carrier for BaP because it adsorbs and strongly binds to PAHs and is not known to be mutagenic or carcinogenic in mammalian systems [27]. Rats that were not exposed served as unexposed controls (UNC) in this study and were subjected to conditions of restraint similar to those received by the rats in the BaP exposure groups. We did not control for the carrier of BaP (CB) because of the lack of effect of this agent on the endocrine and reproductive characteristics of CB-exposed rats [19,23]. Besides, studies conducted by us [28] and others [29] have already established the negligible bioavailability of inhaled BaP associated with CB. This study was approved by the Animal Care and Use Committee of Meharry Medical College, Nashville, TN and conforms to the guidelines of the U.S. EPA (USEPA, [30]) and the European Union [31] for conducting inhalation exposures. Details on the design, fabrication, installation, and characterization of the exposure system are reported in Hood et al. [32]. The methods for aerosol generation, preparation of CB cakes and impactor substrates as well as the characterization and quantification of BaP aerosol and substrate extraction, analysis and quality assurance/control are described in detail in Hood et al. and Inyang et al. [23, 32].
2.2. Post-exposure processing of blood and tissue samples
At the end of the exposure period, rats (n =5/group) were evaluated for the effect of BaP on their ability to cycle regularly using vaginal histologies as described above. Each rat was anesthetized with isoflurane between 1300 and 1500 hr at each detected stage of the estrous cycle to facilitate blood sampling via orbital sinus puncture using heparinized pulled Pasteur pipettes. Plasma was harvested from each blood sample by centrifugation at 1500-x g at 4°C for 10 minutes and stored at −20°C until assayed for ovarian steroids (E2, P4) and gonadotropins (LH and FSH). The above BaP-exposed and UNC rats were subsequently sacrificed by CO2 asphyxiation at the second detected proestrus. Ovaries, samples of liver and lung tissues were thereafter harvested and stored frozen at −80°C until analyzed for BaP metabolite content and Aryl hydrocarbon hydroxylase (AHH) activity.
2.3. Post-exposure fertility evaluation
Benzo(a)pyrene-exposed and UNC rats were placed with vasectomized (n = 5/group) or intact male (n = 10/group) rats of proven fertility for a period of 9 days in a 1:1 ratio to be mated. During their residence with males, female rats were examined daily for evidence of mating based on the presence of mating plug in each rat's vaginal os. On the day of detected mating, female rats mated with vasectomized males were sacrificed by CO2 asphyxiation at 0800 hr. Subsequently, ovaries were excised, trimmed of fat and adherent connective tissues and weighed. Oviducts were dissected out of each animal and placed in a dish containing Armstrong' medium [33] supplemented with 0.1 % hyaluronidase [34]; medium) and the ovulatory pouch poked with a fine needle to expel cumulus masses into the medium. Cumulus masses were allowed to incubate in the medium for 15 min at ambient temperature to facilitate the separation of ovulated eggs from the cumulus cells. Ovulation rate per rat was determined by counting the total number of ovulated eggs recovered from both oviducts and confirmed by the total number of corpora lutea formed from recently ovulated follicles with the aid of a Nikon SMZ stereo zoom dissecting microscope (Japan). Corpora lutea (CLs) that were well perfused with blood, hence their pink color as opposed to those that appeared ischemic, were considered corpora lutea formed from recent ovulations. Female rats mated with intact males were allowed to carry their pregnancy to term. Mean number of pups per litter was calculated by dividing the total number of pups by the number of mated dams. Percentage fetal survival at term was determined as follows: number of pups/number of ovulated eggs from females mated with vasectomized males × 100.
2.4. Radioimmunoassay
Plasma samples were analyzed for E2, P4, LH, and FSH using RIA methods previously validated in our laboratory. The sensitivity of E2 assay was 2 pg/tube and the intra-and inter-assay coefficients of variation (CVs) were 4.9 and 10.8% respectively. The sensitivity of P4 assay was 10 pg/tube and the intra-and inter-assay CVs were 6.8 and 15.8%, respectively. The antisera used in these ovarian steroid assays were donations to our laboratory by Dr. Niswender, Colorado State Univ., Fort Collins, CO. The sensitivity of LH assay was 0.08 ng/tube and the inter- and intra-assay CVs were 6.5 and 10.9%, respectively. Assay sensitivity for FSH was 0.09 ng/tube and the intra- and inter-assay CVs were 4.2 and 11.1%, respectively.
2.5. Extraction and analysis of tissues for benzo(a)pyrene metabolites
Ovarian, liver and lung tissue samples excised from BaP-exposed and UNC rats at proestrus were washed separately in chilled (4°C) isotonic saline to remove excess blood. Subsequently, each of the tissue samples were separately cut into small pieces using sterile scalpel blades following which they were minced with a fine pair of scissors and thoroughly mixed to obtain a homogenous mixture of each minced tissue sample/rat. The samples were weighed and chilled in isotonic saline following which BaP metabolites in the above tissues were extracted, identified and quantified by a reverse-phase HPLC equipped with a UV and a fluorescence detector as detailed in Ramesh et al. [28].
2.6. Aryl hydrocarbon hydroxylase (AHH) assay
Microsomes were isolated from ovarian, liver and lung homogenates as detailed in Ramesh et al. [35]. Microsomal protein concentrations were determined according to the method of Bradford [36]. The AHH activity of these sub-cellular preparations was assayed according to the method of Yang et al. [37].
2.7. Statistical Analyses
Data on length of the estrous cycle, ovarian weight, ovulation rate, number of pups born, BaP metabolite concentrations and AHH activity were analyzed by one-way analysis of variance (ANOVA) and the differences among means tested with orthogonal contrasts. Data on the ability of animals to cycle and percentage fetal survival were analyzed by Chi-Square. Data on hormone concentrations were analyzed by ANOVA with repeated measure and the differences among means tested as described above.
3. Results
3.1. Influence of BaP exposure on the estrous cycle
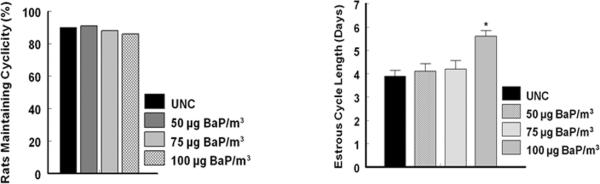
Exposure of rats to different exposure concentrations of BaP did not compromise their ability to cycle compared to their counterparts in the UNC group (Fig. 1A). Interestingly, estrous cycle length of most of the rats exposed to 100 μg/m3 of BaP was extended by approximately 24 hr (P<0.05; Fig. 1B) at the pro-estrus stage of the cycle (Fig. 2) compared to that of animals in the 50, 75 μg/m3 or UNC group. The duration of the other stages of the estrous cycle did not differ among rats in the three BaP exposure groups and UNC.
Fig. 1.
Effect of inhaled BaP on percentage of rats that maintained their reproductive cycle (A) and dose dependent effect of BaP on estrous cycle length (B). Data are presented as percentage (n = 6 animals/treatment group). Regardless of the exposure concentration of BaP rats were exposed to in this study, their ability to cycle was not affected. However, estrous cycle length was increased by about 24 hr in rats exposed to 100 μg BaP/m3 versus UNC or those exposed to lower exposure concentrations of BaP. Data are presented as mean ± SEM (n = 6 animals/treatment group; *p < 0.05).
Fig. 2.
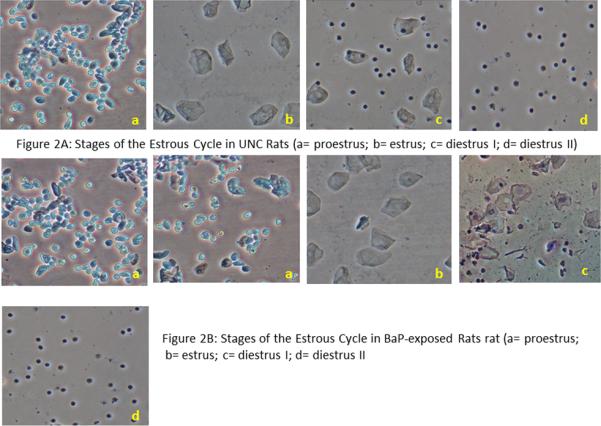
Stages of the estrous cycle in UNC rats (A) and stages of the estrous cycle in rats exposed to BaP (B). Rats in the UNC group exhibited a normal 4-day estrous cycle characteristic of rodents (a= proestrus; b= estrus; c= diestrus I; d= diestrus II; approximately 24 hr /stage of the cycle). While the length of the cycle did not change for rats exposed to 50 and 75 μg BaP/m3, those exposed to 100 μg BaP/m3 sustained an increase in the duration of proestrus from approximately 24 to 48hr.
3.2. Reproductive characteristics of BaP-exposed versus UNC female rats
Table 1 depicts the reproductive characteristics of BaP-exposed versus UNC rats. Ovarian weights tended to be lower in rats in the 3 exposure concentrations of BaP versus those in the UNC group but the difference was not statistically significant (p > 0.05).
Table 1.
Reproductive characteristics of unexposed control and BaP-exposed rats.
| Item | UNC | 50 μg/m3 BaP | 75 μg/m3 BaP | 100 μg/m3 BaP |
|---|---|---|---|---|
| Ovarian Wt (g) | 0.68 ± 0.004 | 0.61 ± 0.003 | 0.59 ± 0.002 | 0.60 ± 0.003 |
| Ovulation Rate | 15.3 ± 2.0 | 13.9 ± 3.0 | 12.8 ± 2.5 | 8.3 ± 1.0* |
| % Ovulated Mature Eggs | 96 | 98 | 96 | 97 |
| Mean Number Of Pups Born (Mean ± SE) | 15.0 ± 4.0 | 13.4 ± 1.0 | 12.3 ± 1.0 | 4.3 ± 2.0** |
| % Fetal Survival | 98.03 | 96.4 | 96.1 | 51.8** |
p < 0.05;
p < 0.002
Even though BaP exposure did not significantly reduce ovarian weights in exposed rats, ovulation rates among BaP-exposed rats decreased in an exposure concentration dependent manner with significant reductions occurring in rats exposed to 100 μg/m3 (p < 0.05) compared with those of rats in the 50, 75 μg/m3 or UNC group. Rats exposed to 50 and 75 μg BaP/m3 had ovulation rates similar to UNC rats. The percentage of ovulated ova that attained nuclear maturation (eggs at MII stage of meiotic maturation) was similar among rats in the different BaP exposure (50μg BaP/m3, 98%; 75 μg BaP/m3, 96%; 100 μg BaP/m3, 97%) groups and UNC (96%) rats. Percentage fetal survival was significantly reduced in rats exposed to 100 μg/m3 BaP before mating compared with comparable fetal survival rates among their counterparts in 50, 75 μg BaP/m3 or UNC group. Consequently, the percentage fetal losses were significantly higher among rats in 100 μg/m3 BaP (48.2%) compared with comparable rates of fetal losses among their counterparts in 50 (3.6%), 75 μg BaP/m3 (3.9%) or UNC (1.96%) group.
3.3. Aryl hydrocarbon hydroxylase activities and disposition of BaP metabolites in plasma and target tissues
The concentrations of BaP metabolites and AHH activities in the organ of BaP entry (lung), the organ of detoxification (liver) and the organ of target action (ovary) of rats exposed via inhalation to sub-acute doses of this PAH are depicted in Table 2. The AHH activities in rats exposed to 50, 75 or 100μg BaP/m3 increased in a dose dependent manner in the above mentioned organs studied, compared with that of UNC rats for these organs. Even though the activity of AHH in all organs studied increased with increasing exposure concentrations of BaP, the differences in the activity of this enzyme in rats exposed to 50 or 75μg/m3 were not statistically different from that of rats in the UNC group. However, at an exposure concentration of 100μg BaP/m3, the activity of this enzyme significantly increased (p < 0.05) in all organs studied compared with those of rats in the 50, 75μg BaP/m3 or UNC group. Similarly, the concentrations of two metabolites (BaP 7,8-diol and BaP 3,6-dione) relevant to toxicity increased in a dose dependent manner in the above mentioned organs studied compared with that of UNC rats. The increases in the concentrations of the above mentioned BaP metabolites for rats exposed to 50 and 75μg BaP/m3 were not statistically different from those of UNC rats for all the organs studied. Interestingly, exposure of rats to 100 μg BaP/m3 significantly increased (p < 0.05) the concentrations of these metabolites in all organs studied compared with those of rats in the 50, 75μg BaP/m3 or UNC group.
Table 2.
Benzo(a)pyrene metabolite concentrations and AHH activities in rats inhalationally exposed to 100μg BaP/m3.
| BaP 7,8-diol¶ (pmol/gm tissue) | BaP 3,6-dione¶ (pmol/gm tissue) | AHH activity (nmol 3(OH) BaP/min/mg protein) | |
|---|---|---|---|
| Ovary | |||
| Control | 0.25 ± 0.03 | 0.42 ± 0.06 | 0.018 ± 0.004 |
| BaP exposed | 12 ± 1.6* | 9.30 ± 0.75* | 0.150 ± 0.032* |
| Lung | |||
| Control | 0.15 ± 0.03 | 0.07 ± 0.01 | 0.017 ± 0.003 |
| BaP exposed | 1.30 ± 0.08* | 1.20 ± 0.01* | 0.260 ± 0.06* |
| Liver | |||
| Control | 0.32 ± 0.02 | 0.22 ± 0.04 | 0.025 ± 0.004 |
| BaP exposed | 3.4 ± 0.2* | 3.00 ± 0.32* | 0.310 ± 0.02* |
p < 0.05
The presence of background concentrations of BaP metabolites in controls arise from inadvertent exposure of these rats to carbon black vapor, the vehicle for B(a)P and which may contain residual levels of BaP. However, the metabolite concentrations measured in controls were low, and the metabolite data for BaP-treated rats were corrected for control values.
3.4. Benzo(a)pyrene alters ovarian steroid and pituitary gonadotropic hormone concentrations
Because 100μg BaP/m3 was the lowest exposure concentration of BaP to effect a significant reduction in ovulation rate, plasma samples from animals in this exposure concentration group (100μg BaP/m3) were used for the determination of the effect of BaP exposure on circulating ovarian steroids (E2 and P4) and gonadotropins (FSH, and LH) at the different stages of the estrous cycle.
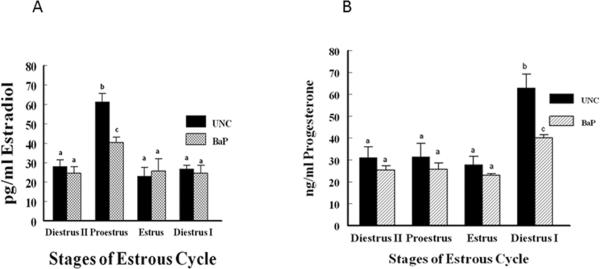
The plasma profile of E2 at the different stages of the estrous cycle studied was similar between BaP-exposed versus UNC rats. However, BaP-exposed rats sustained approximately 34% reduction in plasma concentrations of E2 compared with those of UNC rats at proestrus stage of the estrous cycle (BaP, 40.3 ± 3.0 vs UNC, 61.4 ± 4.3; treatment × stage of the estrous cycle interaction; p < 0.05). The concentrations of this ovarian steroid at estrus, diestrus I and diestrus II did not differ between rats in BaP-exposed and UNC rats (Fig. 3A). Furthermore, the concentrations of P4 in BaP-exposed and UNC rats were comparable at all the stages of the estrous cycle except at diestrus I (Fig. 3B). At the latter stage of the cycle, plasma P4 concentrations among BaP-exposed rats were approximately 44% less (treatment x stage of the estrous cycle interaction p < 0.01) than those of their UNC counterparts. Rats in the UNC group had about 126% increase (p <0.001) in P4 concentrations at diestrus I than at estrus. Similarly, this ovarian steroid also increased by approximately 52% (p < 0.05) in BaP-exposed rats at diestrus I than at estrus but the rate was modest compared with that observed between diestrus I and estrus among UNC rats.
Fig. 3.
Circulating E2 (A) and P4 (B) concentrations in BaP-exposed F-344 adult female rats. Blood samples were collected between 1300 and 1500hr from cycling UNC and BaP-exposed rats at detected stages of the estrous cycle using vaginal smears. The concentrations of E2 and P4 were determined and compared as described in Materials and Methods. Data are presented as mean ± SEM (n = 20 animals/treatment group). Values with different superscripts are significantly different (E2, p < 0.05 stage of the estrous cycle × treatment interaction; P4, b & c versus a, p < 0.01, stage of the estrous cycle × treatment interaction; b versus a, p < 0.001; b versus c, p < 0.05).
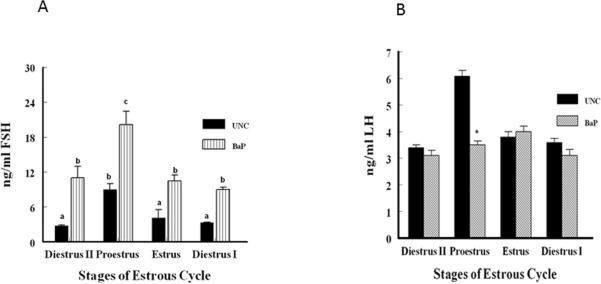
The pattern of plasma FSH secretion among rats in the BaP-exposure group was similar to that of rats in the UNC group during the four stages of the estrous cycle studied, with the highest concentrations occurring at proestrus (p < 0.05). However, plasma FSH concentrations among BaP-exposed rats were significantly higher at all stages of the estrous cycle studied compared to UNC rats (Fig. 4A). The plasma concentrations of FSH among BaP-exposed rats were approximately 3.9, 2.2, 2.6 and 2.7 times those observed in their UNC counterparts (treatment x stages of the estrous cycle interaction; p < 0.05) at diestrus II, estrus, proestrus, and diestrus I stages of the estrous cycle, respectively. The plasma concentrations of LH between BaP-exposed and UNC rats were similar at all stages of the estrous cycle studied except at proestrus (Fig. 4B). As expected, the reduction in plasma estradiol concentrations at proestrus in BaP-exposed rats was accompanied by a concomitant reduction (42%) in circulating concentrations of LH (treatment x stage of the estrous cycle interaction; p < 0.01) compared with those of their UNC counterparts at the same stage of the cycle.
Fig. 4.
Circulating FSH (A) and LH (B) concentrations in BaP-exposed F-344 adult female rats. Blood samples were collected between 1300 and 1500hr from cycling UNC and BaP-exposed rats at detected stages of the estrous cycle using vaginal smears. The concentrations of FSH and LH were determined and compared as described in Materials and Methods. Data are presented as mean ∓ SEM (n = 20 animals/treatment group). FSH values with different superscripts are significantly different (p < 0.05 stage of the estrous cycle × treatment interaction). LH values were higher in plasma of UNC rats at proestrus (*p < 0.05) versus BaP-exposed rats.
4. Discussion
The exposure concentrations of BaP used in this study were chosen based on limits placed by regulatory agencies (National Institute for Occupational Safety and Health [NIOSH], 200 μg PAHs/m3; Occupational Safety and Health Administration [OSHA], 100 μg PAHs/m3 [38]). The common denominator between the exposure concentration limits of PAHs (BaP included) set by NIOSH and OSHA is that they are higher than the lowest exposure concentrations that compromise reproductive function in adult F-344 male and pregnant female rats [19,23,39,40]. These studies suggests that exposures to the aforementioned exposure concentration limits of PAHs set by NIOSH and OSHA still pose a reproductive health hazard to couples aspiring to have children.
Further, the exposure concentrations of BaP used in this study mimic the concentrations in the high exposure scenarios in the general environment. Coke oven workers are exposed to as much as 42 μg BaP/m3 [41]. The average yield of BaP per cigarette varied from 0.96 to 1.9 ng/m3 total particulate matter [42]. A 3-hour stay in pubs exposes occupants to 6.3 ng BaP/m3 released from environmental tobacco smoke [43] with a likelihood of repeated exposure of waitresses and bartenders exposed to this chemical in unrestricted smoking environments in restaurants and taverns. Mean concentrations of BaP in the ambient air of a highly polluted industrial city such as Silesia in Poland have been estimated to crest at about 20 μg/m3 [44]. Furthermore, indoor exposure to BaP from cooking oil fumes in Taiwan has been reported to range between 19 and 23 μg/m3 [45]. Similarly, BaP emitted from wood combustion in rural houses in Burundi were estimated at about 100 μg/m3 [46]. Taken together, human exposures to all the sources of BaP mentioned above in some communities cannot be ruled out and exceed the permissible exposure concentration limits (PECL) set by NIOSH [47], OSHA [48] and ACGIH [49] and pose a serious reproductive health hazard to inhabitants in such communities.
In this study, BaP exposure did not affect the ability of all exposed rats to cycle, however, it extended the length of the estrous cycle by approximately 24hr among rats exposed and 100 μg/m3 compared to their counterparts in the 50, 75μg/m3 or control group. This observation indicates an imbalance in ovarian steroids imposed by BaP and consequently irregular changes in the length of the estrous cycle [50] that occurred at proestrus, a stage of the estrous cycles at which estrogen is maximally secreted by ovarian follicles. We observed a significant reduction in circulating E2 at proestrus among BaP-exposed versus control rats. As a consequence, we hypothesize that the extension of proestrus in BaP-exposed animals is due to a reduction in the level of circulating E2 required to trigger the onset of estrus in a timely manner. The reduced concentrations of E2 at proestrus among BaP-exposed rats reflect a limited number of preantral follicles that progressed to E2-producing antral follicles, hence the low ovulation rate observed in this study among BaP-exposed versus UNC rats. Mattison et al. [7] and Benedict et al. [51] demonstrated that BaP destroys preantral follicles. Based on this premise, we postulate that the number of preantral follicles in ovaries of BaP-exposed rats that could develop to antral follicles and be recruited into the ovulatory pool was limited, hence the reduced number of ovulated eggs in BaP-exposed versus UNC rats. Another contributing factor to the reduced ovulation rate observed among BaP-exposed versus UNC rats is the asynchrony between the developing follicles and preovulatory LH surge. Our data show that plasma LH concentrations increased significantly in the early afternoon of proestrus among UNC rats in a pattern comparable to that observed by Ishigame et al. [52] at proestrus in rats with normal four-day estrous cycle. However, comparable increases in plasma LH concentrations were not observed at the same period during the first day of detected proestrus in BaP-exposed rats. One of the original premise of this study was to compare the endocrine pattern of BaP-exposed rats with those of their UNC counterparts during the different stages of the estrous cycle. Unfortunately we did not evaluate the hormonal patterns of these animals on the second day of detected proestrus in order to observe whether the concentrations of LH observed on the afternoon of the first day of detected proestrus signified a delayed increase in the secretion of this gonadotropin due to exposure to BaP. McLean et al. [53] demonstrated that cigarette smoke, a source of BaP, delays the surge of LH normally observed throughout the afternoon of proestrus. Based on the latter study, an LH surge may have occurred much later in BaP-exposed rats on the second day of detected proestrus because these rats did ovulate albeit fewer eggs compared with UNC rats.
In this study we did not sample the rats frequently enough to detect an LH surge among the UNC rats. However, the significant increase in the concentrations of LH observed in UNC rats suggests that a surge in this gonadotropin was imminent. The surge in LH concentrations in mammals including rats is a functional endocrine event, initiating stages of follicular and oocyte maturation that culminate in the release of an ovum or multiple ova [54]. Based on the low circulating LH levels in BaP-exposed versus UNC rats at day one of detected proestrus, it is highly unlikely that preovulatory follicles in the exposed rats ovulated due to the absence of the type of increases in LH concentrations observed among UNC rats at proestrus. The failure of an LH surge to occur in the afternoon of the first day of detected proestrus could render the preovulatory follicles during this period atretic [55]. The low ovulation rate observed among BaP-exposed versus UNC rats may have resulted from the few healthy preovulatory follicles that were in synchrony with the eventual LH surge in the exposed animals.
The lower systemic concentrations of P4 observed among BaP-exposed rats at diestrus I compared with UNC rats can be attributed to the reduced number of CLs in the former group of rats. It is highly unlikely that the duration of exposure of rats to 100μg BaP/m3 affected progesterone synthesis and release from the corpora lutea. This conclusion is based on the fact that each corpus luteum (CL) among BaP-exposed and UNC rats contributed similar levels of P4 to the general circulation (BaP-exposed, 4.23 ng P4/CL; UNC, 4.12 ng P4/CL).
Our study shows that the percentage of mature ovulated ova was not affected by exposure to BaP, an observation that is in agreement with that made by Sadeu and Foster [56] when primordial follicles were cultured in the presence and absence of BaP under in vitro conditions. The pattern of circulating E2 usually reflects that of E2 in the follicular fluid (FF). High FF E2 concentrations are important markers for follicle and oocyte maturation [57]. The actual concentrations of plasma E2 relative to those in the FF of developing antral follicles is hampered by the lack of knowledge of the rate of metabolism of E2 due to BaP-enhanced phase II metabolic enzyme activation. If follicles in BaP-exposed rats produce E2 at normal levels, it is conceivable that low circulating E2 concentrations at proestrus indicate E2 production by few healthy preovulatory follicles that subsequently expelled mature eggs in synchrony with an LH surge. However, we contend that apparently mature ovulated eggs based on meiotic maturation, are not predictive of subsequent development of generated embryos. Consequently, rats exposed to BaP (100μg/m3) sustained a significantly higher percentage of fetal losses compared with their UNC counterparts. Increased incidence of fetal mortality in BaP-exposed rats and women has been reported by our laboratory [19] and Wu et al. [58], respectively. In the present study, rats were mated post cessation of BaP exposures with unexposed males of established fertility. We speculate that several mechanisms predispose mature ovulated eggs in BaP-treated rats to lower propensity for embryo/fetal generation than those in UNC rats. Since induction of AHH via AhR is vital in metabolizing BaP into reactive and toxic products [25], using the results of AHH activity, we have demonstrated in this study the activation of AhR in the ovaries of exposed animals and the ability of the rat ovary to metabolize BaP into reactive metabolites (BaP 7,8-diol and BaP 3,6-dione). Polycyclic aromatic hydrocarbons including BaP are known to continuously exert their toxic effects long after cessation of exposures due to a long half-life engendered by sequestration in body fat [19] and act through the AhR [25, 59, 60] that is abundantly expressed in oocytes [59]. Activation of AhR induces the expression of genes involved in the metabolism of BaP to active compounds that form DNA adducts [25] and reactive oxygen species (ROS) in granulosa cells beyond the capacity of follicular defense mechanism, hence the repression of E2 synthesis and secretion as discussed below. The reduced litter size among BaP-exposed animals may have resulted from ROS-induced reduction in E2-modulation of the acquisition of oocyte fertilization [61] and post fertilization developmental competence [62]. Oocyte growth and maturation in vivo, are directly regulated by intra-ovarian factors such as steroids, cytokines and other growth factors acting at key points during the process of follicle development [63,64]. Among these factors, E2 may be highly important. Estrogen is required for follicular development and maturation in vivo [65] and also for oocyte fertilization [66–68] in the rabbit, cow and hamster. In the present study follicle maturation in the BaP-treated and control rats occurred in vivo and the plasma E2 concentrations in the former group of rats were below those observed in UNC rats. The observation that the percentage of mature oocytes harvested from BaP-treated rats was comparable with that of their UNC counterparts suggests that oocyte nuclear maturation in rats requires a very low circulating E2 as observed in rhesus monkeys [69]. This being the case, E2 may be important in regulating oocyte cytoplasmic maturation that is essential for the acquisition of oocyte fertilization competence [70].
During implantation, a series of complex interactions exist between the developing blastocysts and the uterus, leading to the establishment of pregnancy [71], processes that are common to many mammalian species. In the rat, implantation is initiated in the evening of day five after fertilization when the blastocysts transit into the uterus. The concerted actions of E2 and P4 via their cognate receptors orchestrate the changes in the uterus that render it competent to permit the attachment of blastocysts and initiation of implantation. It is established that BaP compromises steroidogenesis [19,23,39,40]. Our ongoing study shows that oral exposure of rats to BaP perturbs E2 synthesis by down regulating the aromatase enzyme (data not shown). Furthermore, inhibition of uterine aromatase activity significantly reduces decidual mass which in turn, leads to poor development of implanted embryos and high fetal resorption rates [72]. We contend that BaP contributed to embryo/fetal losses by suppressing uterine aromatase-regulated E2 production. In this study, the concentrations of P4 at diestrus I was reduced in BaP-exposed rats compared to their UNC counterparts while the concentrations of E2 between these groups were comparable at diestrus I. The altered ratio of P4 to E2 in BaP-exposed rats compared with UNC rats may have resulted in an altered uterine secretory activity that was out of synchrony with the stage of embryonic development and thus set a stage for the demise of embryos [73].
High plasma concentrations of FSH in combination with the husbandry system of ad libitum feeding that can induce hyperinsulinemia possibly contributed the higher incidence of embryonic/fetal losses among BaP-exposed versus UNC rats [74]. Eppig et al. [75,76] demonstrated that in vitro culture of oocytes in the presence of FSH and insulin has deleterious effects on pre-implantation development of fertilized ova. It is unlikely that elevated FSH alone in the BaP-exposed rats contributed to impaired oocyte developmental competence in as much as developmentally competent oocytes are produced in vivo in the environment of high circulating FSH in neonatal mice [77,78]. However, elevated plasma insulin in combination with FSH can contribute to oocyte developmental incompetence and offer an explanation to some of the disproportionate fetal losses observed among BaP-exposed versus UNC rats.
Our data [25,79] show that BaP can activate ovarian AhR which, upon heterodimerizing with the AhR nuclear translocator (ARNT) protein, interacts with xenobiotic responsive elements (XREs) transcriptional expressions [80]. Nuclear factor, kappa B (NF-k B), is required for the transcription of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6 and tissue necrosis factor alpha (TNF-α; [81]). Increased TNF-α and IL-6 were correlated with reduced E2 concentrations in follicular fluids of women with immunological infertility compared to women with tubal infertility [82]. Interestingly, we have shown using RT-PCR that TNF-α and IL-6 expressions were significantly increased in the ovaries of rats exposed orally to BaP while circulating E2 concentrations were reduced compared with UNC rats at proestrus (data not shown). The significance of these observations is that BaP can also contribute immunologically to the disruption of E2 secretion at proestrus and subsequent ability of mated BaP-exposed females to support normal embryo/fetal development to term.
The observed decline in fertility indices in rats subjected to sub-acute inhaled BaP serves as a prognosticator for infertility in the event women of reproductive age are exposed to PAHs including BaP. Exposure to PAHs through inhalable particulate matter released from the combustion of biofuels impact negatively on human health in populations in developing countries [83–85] where nearly 2 billion kg of biomass are burned every day [86]. In as much as 50% of the world's households use biofuels for daily cooking and/or heating [86,87], a possibility exists that the preponderant source of exposure is via inhalation of contaminated particulates. Exposure of women and girls to PAHs during cooking and home heating with biomass (wood, charcoal) and fossil (kerosene and liquefied petroleum gas) fuels that are rich in PAHs [88] predisposes them to poor fertility. Furthermore, fumes emitted from cooking oils also contribute significantly to exposure of women to PAHs. A recent study found a significant association between exposure to cooking oil fumes (rich source of PAHs) and cervical intraepithelial neoplasms in women [89], an indication of a dysfunctional organ that is crucial in the reproductive process. Non-smoking Taiwanese women have been reported to be exposed to BaP in the range of 19–23 μg/m3 [90], a level of exposure that significantly exceeds the concentrations reported in cigarette smoke [91] by a factor of 6.5. The long cooking process (1–2 hrs/day) required for preparing fried foods coupled with poor quality oils used in catering facilities equipped with poor ventilation systems predispose women to airborne BaP- and other PAHs-adsorbed particulates every day [92,93]. Furthermore, women who smoke and stir-fry food items on a daily basis are at an increased risk of health hazards [94]. In some cultures, women assume cooking responsibilities soon after marriage (around 20 years of age) until middle age (around 40 to 50 years) when the daughters-in-law subsequently assume their cooking responsibilities [95]. Hence these published reports and dated reported in this study should draw awareness to the reproductive health hazards posed by exposures to BaP and other PAHs to women of reproductive age.
Research Highlights
Exposure of women via inhalation to PAHs including BaP predisposes them to poor fertility and pregnancy outcomes.
A significant decline in fertility indices in adult female rats subjected to sub-acute inhaled BaP was observed.
Our findings suggest that exposure of rats to BaP prior to mating contributes to reduced ovarian function and fetal survival.
Acknowledgments
This work was supported in part by PHS Grants no. U50ATU3989-48-06 (Meharry), 2SO6GMO8037-28 &-32 (AA,AR, DBH), G12RRO3032 (AA & AR), 1U54HD0431501-09 (Meharry), RO1 HD020419-19S1 (AA), 1R15ES012168 (AR), 1RO3CA130112-01 (AR), 1R01CA142845-01A1 (AR), S11ES014156-01 (DBH, AR & AA), and NS41070 (DBH), 5 U54 RR026140-03(NCRR)/ 8 U54 MD007593-03 (NIMHD [SA & AA]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors declare that they have no conflicts of interest.
References
- [1].Abma JC, Chandra A, Mosher WD, Peterson LJ, Piccinino LJ. Fertility, family planning, and women's health: estimates from the 1995 National Survey of Family Growth. Vital Health Stat. 1997;23:19. [PubMed] [Google Scholar]
- [2].Stephen EH, Chandra A. Updated projections of infertility in the United States: 1995–2025. Fertil Steril. 1998;70:30–34. doi: 10.1016/s0015-0282(98)00103-4. [DOI] [PubMed] [Google Scholar]
- [3].Sharara FI, Seifer DB. Flaws JA Environmental toxicants and female reproduction. Fertil Steril. 1998;70:613–622. doi: 10.1016/s0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- [4].Mark-Kappeler CJ, Hoyer PB, Devine PJ. Xenobiotic effects on ovarian preantral follicles. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.091173. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Makuc D, Lalich N. National Center for Health Statistics, DHHS Publication No. 841232. Public Health Service, US Government Printing Office; Washington D.C.: 1983. Employment characteristics of mothers during pregnancy; pp. 25–32. [Google Scholar]
- [6].Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- [7].Mattison DR, White NB, Nightingale MR. The effect of benzo(a)pyrene on fertility, primordial oocyte number, and ovarian response to pregnant mare's serum gonadotropin. Pediat Pharmacol. 1980;1:143–151. [PubMed] [Google Scholar]
- [8].Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- [9].Jick H, Porter J. Relation between smoking and age of natural menopause. Report from the Boston Collaborative Drug Surveillance Program, Boston University Medical Center. Lancet. 1977;1:1354–1355. doi: 10.1016/s0140-6736(77)92562-4. [DOI] [PubMed] [Google Scholar]
- [10].Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. Br Med J (Clin Res Ed) 1985;290:1697–1700. doi: 10.1136/bmj.290.6483.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J, Crosignani PG, Devroey P, Diedrich K, Fauser BC, Fraser L, Glasier A, Liebaers I, Mautone G, Penney G, Tarlatzis B. ESHRE Capri Workshop Group. Fertility and ageing. Hum Reprod Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- [12].Pattinson HA, Taylor PJ, Pattinson MH. The effect of cigarette smoking on ovarian function and early pregnancy outcome of in vitro fertilization treatment. Fertil Steril. 1991;55:780–783. doi: 10.1016/s0015-0282(16)54248-4. [DOI] [PubMed] [Google Scholar]
- [13].Seltzer V. Smoking as a risk factor in the health of women. Int J Gynaecol Obstet. 2003;82:393–397. doi: 10.1016/s0020-7292(03)00227-3. [DOI] [PubMed] [Google Scholar]
- [14].Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10:3213–3217. doi: 10.1093/oxfordjournals.humrep.a135891. [DOI] [PubMed] [Google Scholar]
- [15].Neal MS, Hughes EG, Holloway AC, Foster WG. Sidestream smoking is equally as damaging as mainstream smoking on IVF outcomes. Hum Reprod. 2005;20:2531–2535. doi: 10.1093/humrep/dei080. [DOI] [PubMed] [Google Scholar]
- [16].Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. (Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem Toxicol. 2002;40:93–104. doi: 10.1016/s0278-6915(01)00085-0. [DOI] [PubMed] [Google Scholar]
- [17].Lodovici M, Akpan V, Evangelisti C, Dolara P. Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J Appl Toxicol. 2004;24:277–281. doi: 10.1002/jat.992. [DOI] [PubMed] [Google Scholar]
- [18].Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update. 2000;6:122–131. doi: 10.1093/humupd/6.2.122. [DOI] [PubMed] [Google Scholar]
- [19].Archibong AE, Inyang F, Ramesh A, Greenwood M, Nayyar T, Kopsombut P, Hood DB, Nyanda AM. Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed by inhalation to benzo(a)pyrene. Reprod. Toxicol. 2002;16:801–808. doi: 10.1016/s0890-6238(02)00058-8. [DOI] [PubMed] [Google Scholar]
- [20].Ramesh A, Archibong A. Reproductive toxicity of polycyclic aromatic hydrocarbons: occupational relevance. In: Gupta RC, editor. Reproductive and Developmental Toxicology. Academic Press; London: 2011. pp. 577–592. [Google Scholar]
- [21].Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider H, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol. 2004;23:301–33. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- [22].Charles GD, Bartels MJ, Zacharewski TR, Gollapudi BB, Freshour NL, Carney EW. Activity of benzo(a)pyrene and its hydroxylated metabolites in an estrogen receptor-α reporter gene assay. Toxicol. Sci. 2000;55:320–326. doi: 10.1093/toxsci/55.2.320. [DOI] [PubMed] [Google Scholar]
- [23].Inyang F, Ramesh A, Kopsombut P, Niaz MS, Hood DB, Nyanda AM, Archibong AE. Disruption of testicular steroidogenesis and epididymal function by inhaled benzo(a)pyrene. Reprod Toxicol. 2003;17:527–537. doi: 10.1016/s0890-6238(03)00071-6. [DOI] [PubMed] [Google Scholar]
- [24].Kim J-C, Kim S-H, Shin D-H, Ahn T-H, Kim H-C, Kim Y-B, Jiang C-Z, Han J, Chung M-K. Effects of prenatal exposure to the environmental pollutant 2-bromopropane on embryo-fetal development in rats. Toxicology. 2004;196:77–86. doi: 10.1016/j.tox.2003.11.006. [DOI] [PubMed] [Google Scholar]
- [25].Ramesh A, Archibong AE, Niaz MS. Ovarian susceptibility to benzo(a)pyrene: tissue burden of metabolites and DNA adducts in F-344 rats. J Toxicol Environ Health A. 2010;73:1611–1625. doi: 10.1080/15287394.2010.514225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- [27].Accardi-Dey A, Gschwend PM. Reinterpreting literature sorption data considering both absorption into organic carbon and adsorption onto black carbon. Environ Sci Technol. 2003;37:99–106. doi: 10.1021/es020569v. [DOI] [PubMed] [Google Scholar]
- [28].Ramesh A, Greenwood M, Inyang F, Hood DB. Toxicokinetics of inhaled benzo(a)pyrene: plasma and lung bioavailability. Inhalat Toxicol. 2001;13:533–553. doi: 10.1080/08958370118859. [DOI] [PubMed] [Google Scholar]
- [29].Wolff RK, Bond JA, Sun JD, Henderson RF, Harkema JR, Griffith WC, et al. Effects of adsorption of benzo(a)pyrene onto carbon black particles on levels of DNA adducts in lungs of rats exposed by inhalation. Toxicol. Appl. Pharmacol. 1989;97:289–299. doi: 10.1016/0041-008x(89)90334-7. [DOI] [PubMed] [Google Scholar]
- [30].US Environmental Protection Agency Acute Inhalation Toxicity. TSCA 798; 40 CFR 790. 1989 [Google Scholar]
- [31].European Union Guideline 92/69/ECC Acute toxicity inhalation. Office J Eur Comm Legal Spec L383A. 1992;35:117. [Google Scholar]
- [32].Hood DB, Nayyar T, Ramesh A, Greenwood M, Inyang F. Modulation in the developmental expression profile of SP1 subsequent to transplacental exposure of fetal rats to desorbed Benzo(a)pyrene following maternal inhalation. Inhalat. Toxicol. 2000;12:511–535. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- [33].Armstrong DT, Zhang X, Vanderhyden BC, Khamsi F. Hormonal actions during oocyte maturation influence fertilization and early embryonic development. Ann N Y Acad Sci. 1991;626:137–158. doi: 10.1111/j.1749-6632.1991.tb37908.x. [DOI] [PubMed] [Google Scholar]
- [34].Yanagimachi R, Yanagimachi H, Rogers BJ. The use of zona-free animal ova as a test-system for the assessment of the fertilizing capacity of human spermatozoa. Biol Reprod. 1976;15:471–476. doi: 10.1095/biolreprod15.4.471. [DOI] [PubMed] [Google Scholar]
- [35].Ramesh A, Inyang F, Hood DB, Knuckles ME. Aryl hydrocarbon hydroxylase activity in F-344 rats subchronically exposed to benzo(a)pyrene and fluoranthene through diet. J Biochem Mol Toxicol. 2000;14:155–161. doi: 10.1002/(sici)1099-0461(2000)14:3<155::aid-jbt5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [36].Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein, utilizing the principle of dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- [37].Yang S-C, Jenq S-N, Kang ZC, Lee H. Identification of benzo(a)pyrene 7,8-diol 9,10-epoxide N2-deoxyguanosine in human lung adenocarcinoma cells exposed to cooking oil fumes from frying fish under domestic conditions. Chem Res Toxicol. 2000;13:1046–1050. doi: 10.1021/tx0000419. [DOI] [PubMed] [Google Scholar]
- [38].ATSDR . Toxicological profile for polycyclic aromatic hydrocarbons (PAHs) Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services, US Public Health Service; Atlanta, GA: 1995. p. 271. [Google Scholar]
- [39].Ramesh A, Inyang F, Lunstra DD, Niaz MS, Kopsombut P, Jones KM, Hood DB, Hills ER, Archibong AE. Alteration of fertility endpoints in adult male F-344 rats by subchronic exposure to inhaled benzo(a)pyrene. Exp Toxic Pathol. 2008;60:269–280. doi: 10.1016/j.etp.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Archibong AE, Ramesh A, Niaz MS, Brooks CM, Roberson SI, Lunstra DD. Effects of benzo(a)pyrene on intratesticular function in F-344 rats. Int J Environ Res Public Health. 2008;5:32–40. doi: 10.3390/ijerph5010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lewtas J, Walsh D, Williams R, Dobias L. Air pollution exposure-DNA adduct dosimetry in humans and rodents: evidence for non-linearity at high doses. Mutat Res. 1997;378:51–63. doi: 10.1016/s0027-5107(97)00097-3. [DOI] [PubMed] [Google Scholar]
- [42].Li S, Olegario RM, Banyasz JL, Shafer KH. Gas chromatography-mass spectrometry analysis of polycyclic aromatic hydrocarbons in single puff of cigarette smoke. J Anal Appl Pyrolysis. 2003;66:155–163. [Google Scholar]
- [43].Besaratinia A, Maas LM, Brouwer EMC, Moonen EJC, Dekok TMCM, Wesseling GJ, Loft S, Kleinjans JCS, van Schooten FJ. A molecular dosimetry approach to assess human exposure to environmental topbacco smoke in pubs. Carcinogenesis. 2002;23:1171–1176. doi: 10.1093/carcin/23.7.1171. [DOI] [PubMed] [Google Scholar]
- [44].Chorazy M, Szeliga J, Strozyk M, Cimander B. Ambient air pollutants in upper Silesia: partial chemical composition and biological activity. Environ Health Perspect. 1994;102(Suppl 4):61–66. doi: 10.1289/ehp.94102s461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chiang T-A, Wu P-F, Ko-Y-C Identification of carcinogens in cooking oil fumes. Environ Res A. 1999;81:18–22. doi: 10.1006/enrs.1998.3876. [DOI] [PubMed] [Google Scholar]
- [46].Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occup Environ Health. 2000;73:331–338. doi: 10.1007/s004209900112. [DOI] [PubMed] [Google Scholar]
- [47].National Institute for Occupational Safety and Health (NIOSH) Pocket Guide to Chemical Hazards. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention; Cincinnati, OH: 1997. [Google Scholar]
- [48].Occupational Safety and Health Administration (OSHA) Occupational Safety and Health Standards, Toxic and Hazardous Substances. Code of Federal Regulations. 1998;29 CFR 1910.1000. [Google Scholar]
- [49].American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Values for Chemical Substances and Physical Agents, Biological Exposure Indices. Cincinnati, OH: 1999. 1999 TLVs and BEIs. [Google Scholar]
- [50].Shivalingappa H, Satyanarayan ND, Purohit MG, Sharanabasappa A, Patil SB. Effect of ethanol extract of Rivea hypocrateriformis on the estrous cycle of the rat. J Ethnopharmacol. 2002;82:11–17. doi: 10.1016/s0378-8741(02)00073-9. [DOI] [PubMed] [Google Scholar]
- [51].Benedict JC, Miller KP, Lin TM, Greenfeld C, Babus JK, Peterson RE, Flaws JA. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod. 2003;68:1511–1517. doi: 10.1095/biolreprod.102.007492. [DOI] [PubMed] [Google Scholar]
- [52].Ishigame H, Medan MS, Watanabe G, Shi Z, Kishi H, Arai KY, Taya K. A new alternative method for superovulation using passive immunization against inhibin in adult rats. Biol Reprod. 2004;7:236–243. doi: 10.1095/biolreprod.104.027789. [DOI] [PubMed] [Google Scholar]
- [53].McLean BK, Rubel A, Nikitovitch-Winer MB. The differential effects of exposure to tobacco smoke on the secretion of luteinizing hormone and prolactin in the proestrous rat. Endocrinology. 1977;100:1566–1570. doi: 10.1210/endo-100-6-1566. [DOI] [PubMed] [Google Scholar]
- [54].Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, Leranth C, Vondracek-Klepper S, Lewis C, Chang A, Parducz A. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- [55].Peluso JJ, Steger RW, Hafez ES. Regulation of LH secretion in aged female rats. Biol Reprod. 1977;16:212–215. doi: 10.1095/biolreprod16.2.212. [DOI] [PubMed] [Google Scholar]
- [56].Sadeu JC, Foster WG. Effect of in vitro exposure to benzo[a]pyrene, a component of cigarette smoke, on folliculogenesis, steroidogenesis and oocyte nuclear maturation. Reprod Toxicol. 2011;31:402–408. doi: 10.1016/j.reprotox.2010.12.006. [DOI] [PubMed] [Google Scholar]
- [57].Pellicer A, Lightman A, Diamond MP, Russell JB, DeCherney AH. Outcome of in vitro fertilization in women with low response to ovarian stimulation. Fertil Steril. 1987;47:812–815. doi: 10.1016/s0015-0282(16)59170-5. [DOI] [PubMed] [Google Scholar]
- [58].Wu J, Hou H, Ritz B, Chen Y. Exposure to polycyclic aromatic hydrocarbons and missed abortion in early pregnancy in a Chinese population. Sci Total Environ. 2010;408:2312–2318. doi: 10.1016/j.scitotenv.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sun F, Betzendahl I, Shen Y, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Preantral follicle culture as a novel in vitro assay in reproductive toxicology testing in mammalian oocytes. Mutagenesis. 2004;19:13–25. doi: 10.1093/mutage/geg040. [DOI] [PubMed] [Google Scholar]
- [60].Uno S, Endo K, Ishida Y, Tateno C, Makishima M, Yoshizato K, Nebert DW. CYP1A1 and CYP1A2 expression: comparing 'humanized' mouse lines and wild-type mice; comparing human and mouse hepatoma-derived cell lines. Toxicol Appl Pharmacol. 2009;237:119–126. doi: 10.1016/j.taap.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zelinski-Wooten MB, Hess DL, Baughman WL, Molskness TA, Wolf DP, Stouffer RL. Administration of an aromatase inhibitor during the late follicular phase of gonadotrophin-treated cycles in rhesus monkeys: effects on follicle development, oocyte maturation, and subsequent luteal function. J Clin Endocrinol Metab. 1993;76:988–995. doi: 10.1210/jcem.76.4.8473415. [DOI] [PubMed] [Google Scholar]
- [62].Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alpha-glucoside during in vitro maturation. Biol Reprod. 2001;65:1800–1806. doi: 10.1095/biolreprod65.6.1800. [DOI] [PubMed] [Google Scholar]
- [63].Campbell BK, McNeilly AS. Follicular dominance and oocyte maturation. Zygote. 1996;4:327–334. doi: 10.1017/s0967199400003348. [DOI] [PubMed] [Google Scholar]
- [64].Fulvio C. Intra-ovarian regulation of oocyte developmental competence in cattle. Zygote. 1996;4:323–326. doi: 10.1017/s0967199400003336. [DOI] [PubMed] [Google Scholar]
- [65].Richards JS, Jahnsen T, Hedin L, Lifka J, Ratoosh S, Durica JM, Goldring NB. Ovarian follicular development: from physiology to molecular biology. Recent Prog Horm Res. 1987;43:231–276. doi: 10.1016/b978-0-12-571143-2.50012-5. [DOI] [PubMed] [Google Scholar]
- [66].Suzuki S, Endo Y, Tanaka S, Iizuka R. Indirect immunofluorescence studies on the steroid-producing activity of hamster ova. Am J Obstet Gynecol. 1984;148:76–85. doi: 10.1016/s0002-9378(84)80036-8. [DOI] [PubMed] [Google Scholar]
- [67].Yoshimura Y, Hosoi Y, Atlas SJ, Bongiovanni AM, Santulli R, Wallach EE. The effect of ovarian steroidogenesis on ovulation and fertilizability in the in vitro perfused rabbit ovary. Biol Reprod. 1986;35:943–948. doi: 10.1095/biolreprod35.4.943. [DOI] [PubMed] [Google Scholar]
- [68].Saeki K, Kato H, Hosoi Y, Miyake M, Utsumi K, Iritani A. Early morphological events of in vitro fertilized bovine oocytes with frozen-thawed spermatozoa. Theriogenology. 1991;35:1051–1058. doi: 10.1016/0093-691x(91)90314-4. [DOI] [PubMed] [Google Scholar]
- [69].Zheng P, Wei S, Bavister BD, Yang J, Ding C, Ji W. 17b-Estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod. 2003;18:2137–2144. doi: 10.1093/humrep/deg410. [DOI] [PubMed] [Google Scholar]
- [70].Zelinski-Wooten MB, Hess DL, Wolf DP, Stouffer RL. Steroid reduction during ovarian stimulation impairs oocyte fertilization, but not folliculogenesis, in rhesus monkeys. Fertil Steril. 1994;61:1147–1155. [PubMed] [Google Scholar]
- [71].Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–37. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- [72].Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci U S A. 2009;106:12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Archibong AE, England DC, Stormshak F. Factors contributing to early embryonic mortality in gilts bred at pubertal estrus. J Anim Sci. 1987;64:474–478. doi: 10.2527/jas1987.642474x. [DOI] [PubMed] [Google Scholar]
- [74].Kanety H, Moshe S, Shafrir E, Lunenfeld B, Karasik A. Hyperinsulinemia induces a reversible impairment in insulin receptor function leading to diabetes in the sand rat model of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1994;91:1853–1857. doi: 10.1073/pnas.91.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- [76].Eppig JJ, Hosoe M, O'Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163:109–116. doi: 10.1016/s0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- [77].Dullaart J, Kent J, Ryle M. Serum gonadotrophin concentrations in infantile female mice. Reprod Fertil. 1975;43:189–192. doi: 10.1530/jrf.0.0430189. [DOI] [PubMed] [Google Scholar]
- [78].Halpin DM, Charlton HM, Faddy MJ. Effects of gonadotrophin deficiency on follicular development in hypogonadal (hpg) mice. Reprod Fertil. 1986;78:119–25. doi: 10.1530/jrf.0.0780119. [DOI] [PubMed] [Google Scholar]
- [79].Harris DL, Huderson AC, Niaz MS, Ford JJ, Archibong AE, Ramesh A. Comparative metabolism of benzo(a)pyrene by ovarian microsomes of various species. Environ Toxicol. 2009;24:603–609. doi: 10.1002/tox.20461. [DOI] [PubMed] [Google Scholar]
- [80].Antonsson C, Arulampalam V, Whitelaw ML, Pettersson S, Poellinger L. Constitutive function of the basic helix-loop-helix/PAS factor Arnt. Regulation of target promoters via the E box motif. J Biol Chem. 1995;270:13968–13972. doi: 10.1074/jbc.270.23.13968. [DOI] [PubMed] [Google Scholar]
- [81].Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- [82].Calogero AE, Nicoletti F, Palumbo MA, Burrello N, Di Mauro M, Lunetta M, Bendtzen K, Cianci A. Macrophage-derived cytokines in the follicular fluids of women with infertility due to immunological causes. Elevated levels of interleukin 6 and low levels of granulocyte-macrophage colony-stimulating factor. Cytokine. 1998;10:814–818. doi: 10.1006/cyto.1998.0363. [DOI] [PubMed] [Google Scholar]
- [83].Albalak R, Keeler GJ, Frisancho AR, Haber M. Assessment of PM10 concentrations from domestic biomass fuel combustion in rural Bolivian highland villages. Environ Sci Technol. 1999;33:2505–2509. [Google Scholar]
- [84].Ezzati M, Saleh H, Kammen DM. The contributions of emissions and spatial microenvironments to exposure to indoor air pollution from biomass combustion in Kenya. Environ. Health Perspect. 2000;108:833–839. doi: 10.1289/ehp.00108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Balakrishnan K, Parikh J, Sankar S, Padmavathi R, Srividya K, Venugopal V, Prasad S, Pandey VL. Daily average exposures to respirable particulate matter from combustion of biomass fuels in rural households of southern India. Environ Health Perspect. 2002;110:1069–1075. doi: 10.1289/ehp.021101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Reddy AKN, Williams RH, Johansson TB. Energy After Rio: Prospects and Challenges. United Nations Publications; NewYork: 1996. [Google Scholar]
- [87].Oanh N, Reutergardh L, Dung N. Emission of polycyclic aromatic hydrocarbons and particulate matter from domestic combustion of selected fuels (1999) Environ Sci Technol. 1999;33:2703–2709. [Google Scholar]
- [88].Zhang J, Smith KR. Hydrocarbon emissions and health risks from cook stoves in developing countries. J Expo Anal Environ Epidemiol. 1996;6:147–161. [PubMed] [Google Scholar]
- [89].Wu M-T, Lee L-H, Ho C-K, Wu S-C, Lin L-Y, Cheng B-H, Liu C-L, Yang C-Y, Tsai H-T, Wu T-N. Environmental exposure to cooking oil fumes and cervical intraepithelial neoplasm. Environ Res. 2004;94:25–32. doi: 10.1016/s0013-9351(03)00118-x. [DOI] [PubMed] [Google Scholar]
- [90].Chiang TA, Wu PF, Ko YC. Identification of carcinogens in cooking oil fumes. Environ Res. 1999;81:18–22. doi: 10.1006/enrs.1998.3876. [DOI] [PubMed] [Google Scholar]
- [91].Guerrin MR. Tobacco Smoke. Springer-Verlag; NewYork: 1993. Environmental tobacco smoke exposure assessment. [Google Scholar]
- [92].Shuguang L, Dinhua P, Guoxiong W. Analysis of polycyclic aromatic hydrocarbons in cooking oil fumes. Arch. Environ.Hlth. 1994;49:119–122. doi: 10.1080/00039896.1994.9937464. [DOI] [PubMed] [Google Scholar]
- [93].Yang SC, Jenq SN, Kang ZC, Lee H. Identification of benzo[a]pyrene 7,8-diol 9,10-epoxide N2-deoxyguanosine in human lung adenocarcinoma cells exposed to cooking oil fumes from frying fish under domestic conditions. Chem Res Toxicol. 2000;13:1046–1050. doi: 10.1021/tx0000419. [DOI] [PubMed] [Google Scholar]
- [94].Seow A, Poh WT, The M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Fumes from meat cooking and lung cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2000;9:1215–1221. [PubMed] [Google Scholar]
- [95].Wu MT, Lee LH, Ho CK, Wu SC, Lin LY, Cheng BH, Liu CL, Yang CY, Tsai HT, Wu TN. Environmental exposure to cooking oil fumes and cervical intraepithelial neoplasm. Environ Res. 2004;94:25–32. doi: 10.1016/s0013-9351(03)00118-x. [DOI] [PubMed] [Google Scholar]