Figure 10. Models of HEDS cellular reactions.
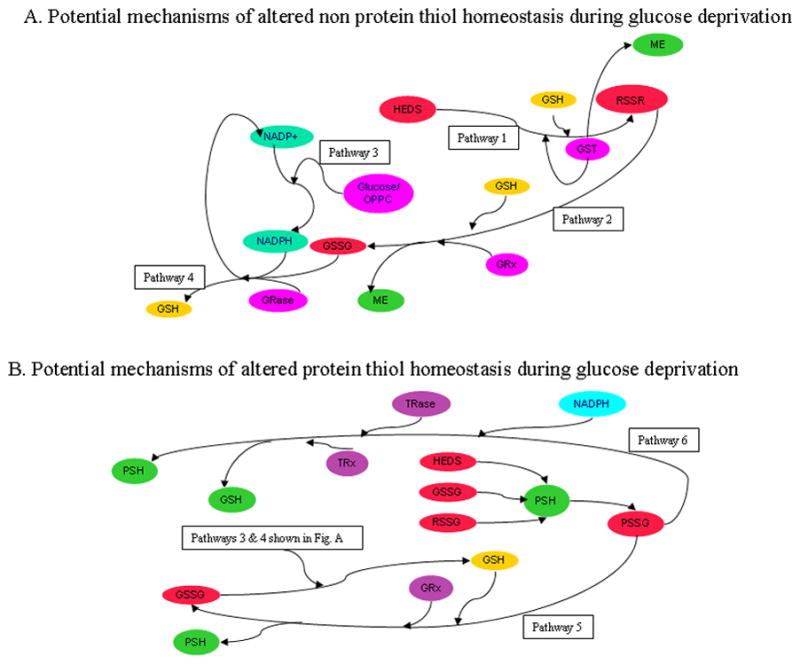
The ability of HEDS to interfere with thiol redox homeostasis and elicit loss of protein function and cell death occurs in glucose-deprived cells where HEDS detoxification is inefficient (Pathways 1–6). (A.) HEDS effect on non-protein thiols (NPSH). Bioreductive/detoxification pathways are similarly active in cells receiving adequate glucose independent of p53 status. HEDS may react spontaneously with GSH or in a reaction catalyzed by glutathione-S-transferase (GST) may produce non protein disulfides (RSSR including GSSG and/or mixed disulfide (MESSG) of glutathione (GSH)) and mercaptoethanol (ME) (Pathway 1). MESSG, if produced, may also react with GSH to produce ME and oxidized GSH (GSSG) through the catalytic action of glutaredoxin (GRX) (Pathway 2). In contrast, glucose-deprived cells convert HEDS into ME with poor efficiency, demonstrating that reductants needed to detoxify HEDS require intracellular glucose (Pathway 3). GSH loss in glucose-depleted cells is likely caused by reduced activity of the oxidative pentose phosphate cycle, a crucial factor in GSH homeostasis by recycling oxidized GSSG back to GSH via glutathione reductase (GRase) and NADPH (Pathway 4). (B). HEDS effect on protein thiols. HEDS, GSSG and/or HEDS-glutathione mixed disulfide (MESSG) produced by HEDS can react with protein thiols (PSH). Oxidation of PSH and loss of Ku function in glucose-depleted cells argues that oxidative pentose phosphate cycle (Pathways 3 and 4) is a crucial pathway for repair of oxidized protein thiols, either through the action of thioredoxin reductase (TRase)/thioredoxin (TRx)/NADPH (Pathway 5) or glutaredoxin (GRx) and GSH (Pathway 6).
