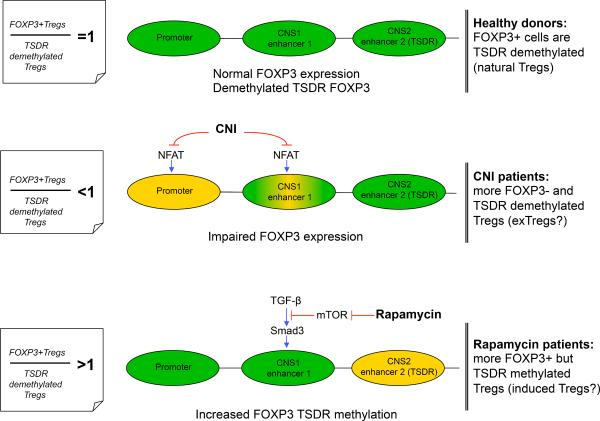
Figure 5. Basis for different FOXP3/TSDR ratios in Tregs from CNI and Rapamycin groups.
To initiate FOXP3 gene transcription, the FOXP3 promoter and at least 1 of 2 enhancer elements must be activated. In natural Tregs, enhancer 2 (TSDR) is demethylated, resulting in sustained FOXP3 expression. In peripherally induced Tregs, TGF-β sensitive enhancer is activated, leading to transient FOXP3 expression. Top: in healthy donors, most Tregs are natural thymic-derived cells expressing FOXP3 and demethylated at the TSDR. Middle: CNI exposure disrupts NFAT signaling, leading to impaired FOXP3 promoter activation (14) and, as a result, leads to an increased percent of TSDR-demethylated (in CNS2 region) cells that are unable to activate FOXP3 transcription, within CD4+CD25+ Tregs. The enhancer 1 region may also be sensitive to CNI, since TGF-β activated induction occurs through the cooperation of NFAT and Smad3 and that can lead to further aggravation of impaired FOXP3 expression in Tregs. Bottom: Activation of enhancer 1 through TGF-β-dependent and TGF-β-independent pathways (the latter not shown in schematic) can be disrupted by AKT-mTOR activity and inhibited by rapamycin, resulting in increased numbers of FOXP3+ peripherally induced Tregs with TSDR-methylated FOXP3. Green regions are active, yellow regions are partially inactive.