Fig. 4. Proteasome inhibition increases the levels of phosphorylated Akt while rapamycin augments an OA-induced increase in ubiquitinated proteins.
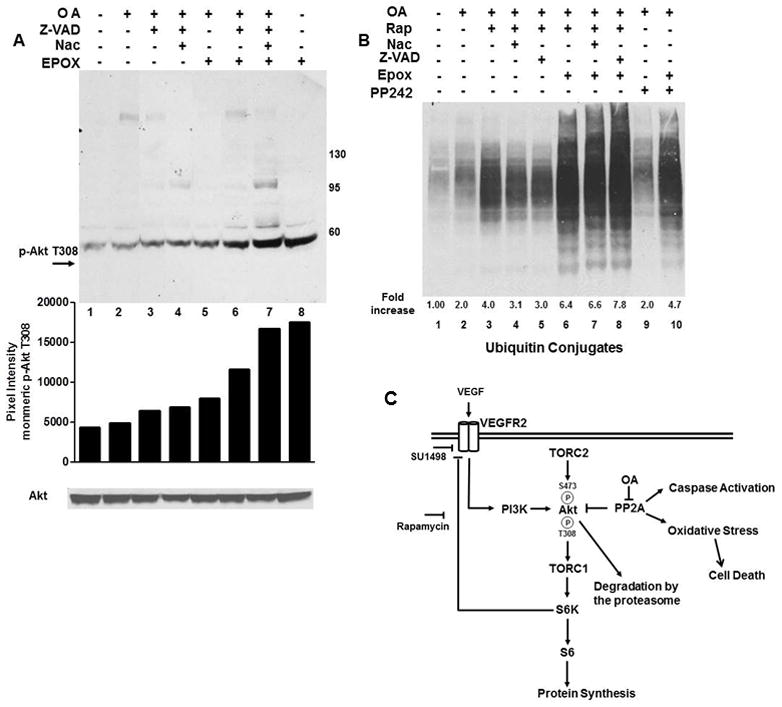
(A) OA treated cells were incubated with Nac, Z-VAD-FMK or 100 nM epoxomicin for 2 hr as indicated. Immunoblots (light exposure) were probed for phosphorylation of monomeric Akt at T308 or total Akt. (B) Cells were treated with OA and with rapamycin, Nac, PP242 or Z-VAD-FMK alone or in combination with epoxomicin and analyzed for ubiquitinated conjugates. Data in A and B were quantified using ImageJ and expressed as relative pixel intensity in A and the fold increase relative to control in B. (C) Model of mTOR, PP2A and VEGFR2 crosstalk in serum starved SK-N-SH cells. OA exacerbates the VEGF/VEGFR2-directed phosphorylation of S6K1 and S6 and Akt phosphorylation at T308 and S473 and induces an oxidative stress-induced cell death. PP2A and mTOR negatively control Akt phosphorylation through dephosphorylation and a S6K1 directed negative feedback of VEGFR2 signaling through PI3K, respectively.
