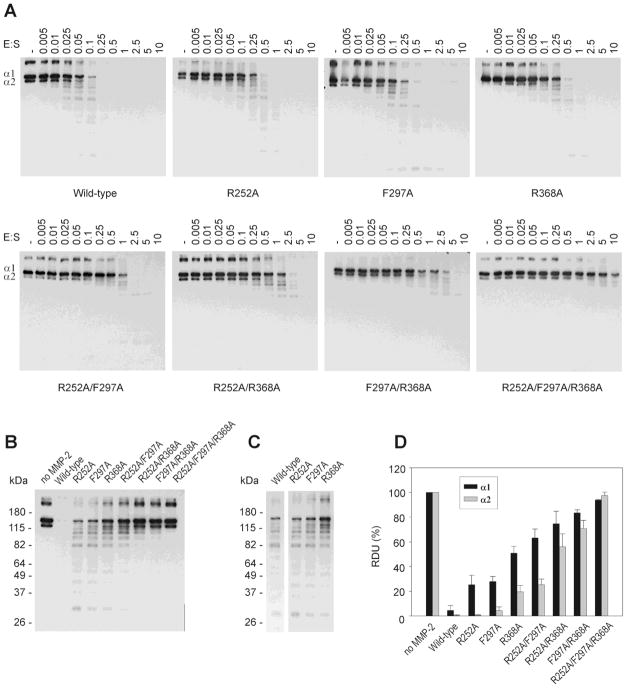
Fig. 6.
Effects of single, double and triple point collagen binding site modifications in the CBD of MMP-2 on the cleavage of α1 and α2 chains in denatured type I collagen. (A) Biotinylated denatured type I collagen (0.025 pmol) was incubated without enzyme (control) or with a concentration range of wild-type and variant MMP-2 (0 – 0.25 pmol) for 20 min at 28°C. The reactions were stopped by adding 2 × sample buffer and separated by SDS/PAGE on 8% gels. The collagen bands were detected by Western blotting using HRP-conjugated streptavidin and ECL as substrate. (B) Equal amounts of biotinylated denatured type I collagen (0.025 pmol) were incubated with wild-type or MMP-2 variants at a constant 1:10 enzyme:substrate ratio as described above. (C) Fragmentation pattern from cleavage of denatured type I collagen by MMP-2 WT and variants with single-point alanine substitutions in each of the three CBD modular collagen binding sites. (D) Quantification of the intensities of the α1 and α2 chains of type I collagen (Panel B) from radiographic films using Kodak 1D imaging software. Presented are means and S.D. expressed relative to untreated control collagen (n = 3).