Abstract
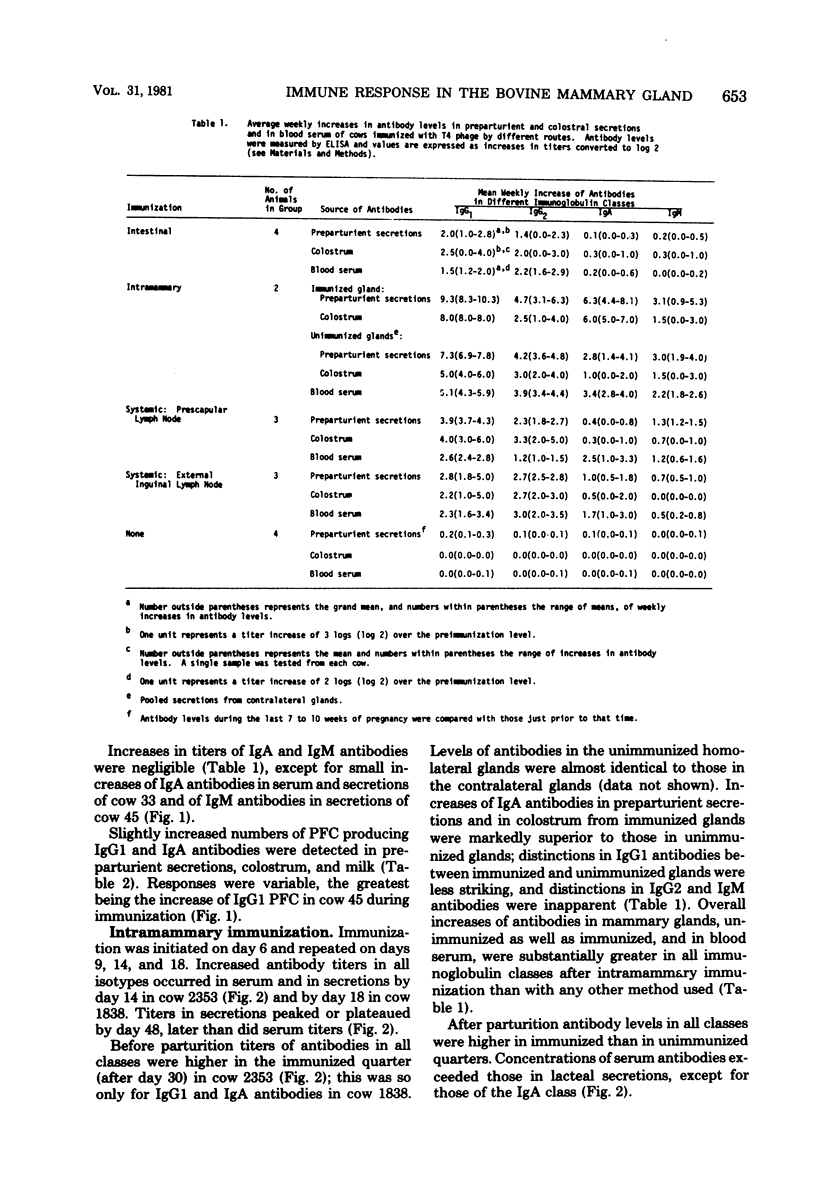
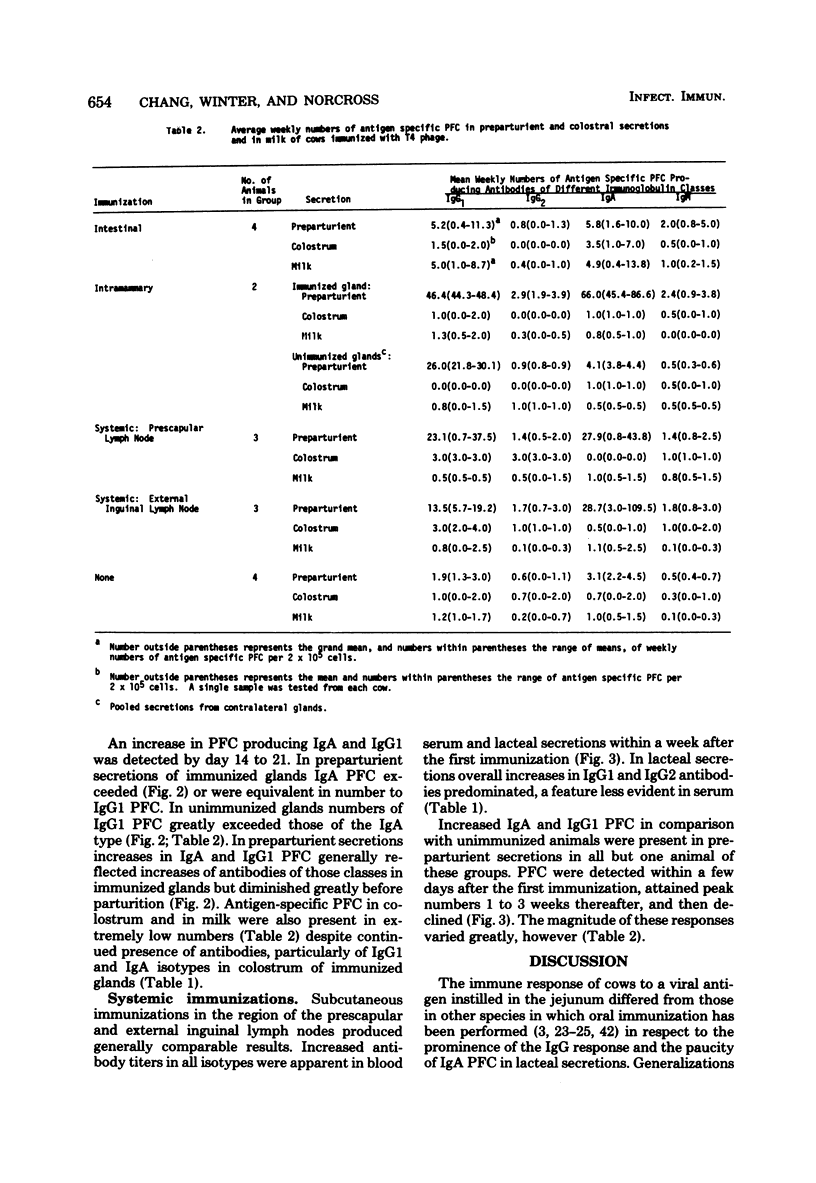
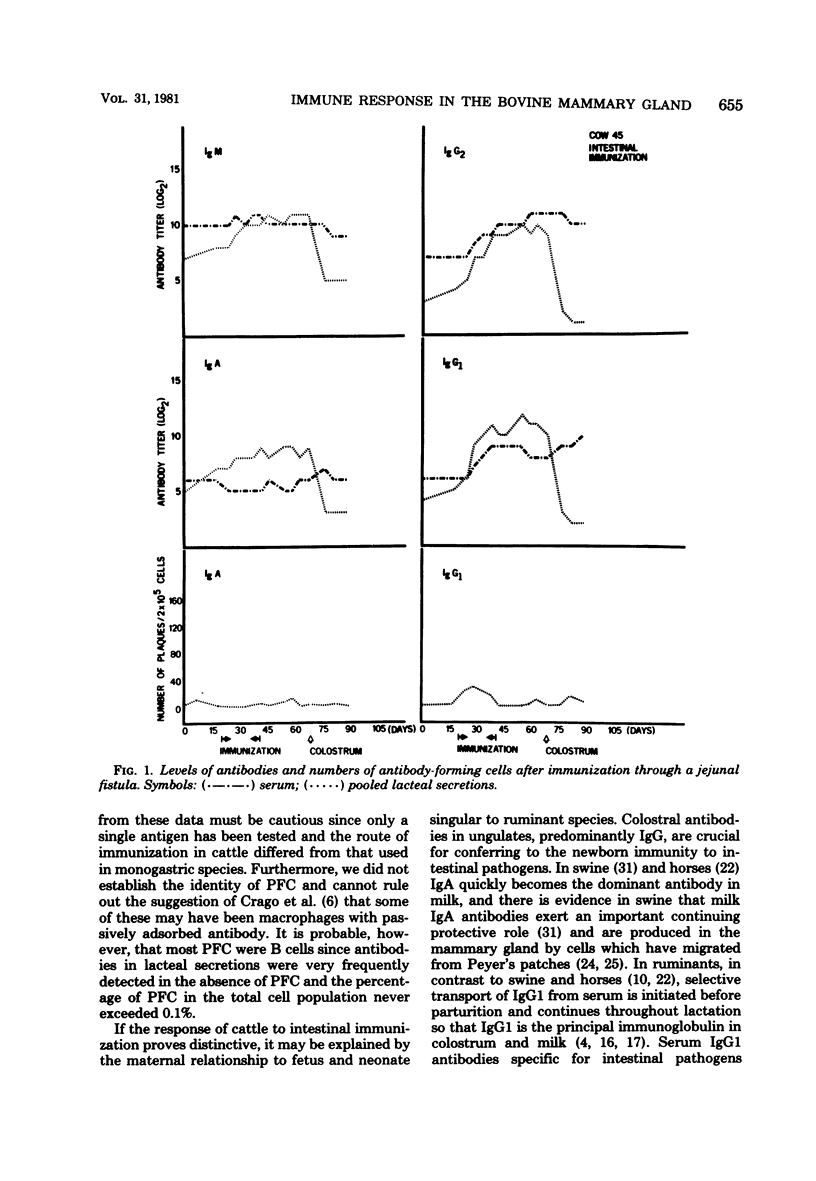
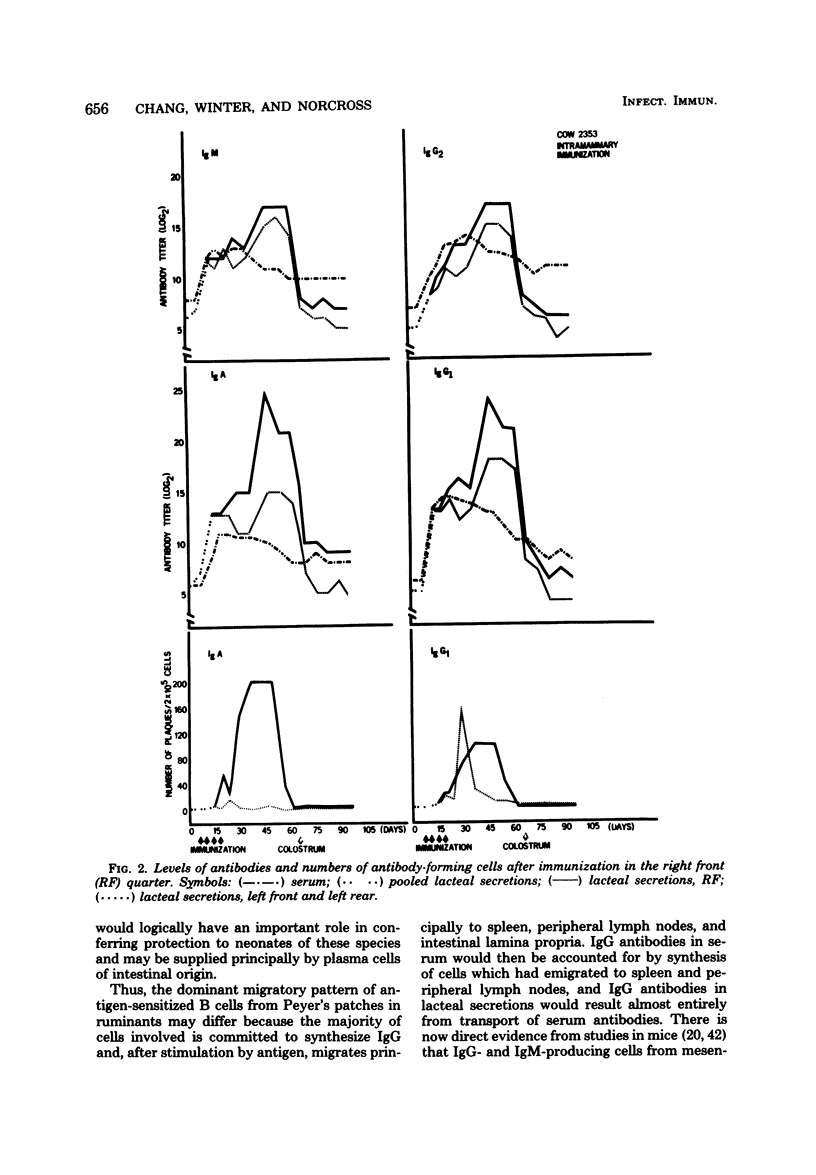
The immune response in mammary glands of cattle was measured after intestinal, local, and systemic immunization with T4 bacteriophage. Nonlactating pregnant cows were immunized by infusions into the intestine or mammary gland and by subcutaneous injections in the region of the prescapular or external inguinal lymph nodes. Titers of antibodies of different isotypes were measured in serum and in lacteal secretions by enzyme-linked immunosorbent assay, and numbers of cells producing antibodies of each isotype were determined in lacteal secretions by the Jerne plaque assay. Substantial increases in immunoglobulin G subclass 1 (IgG1) and IgG2 antibody titers were detected in serum and lacteal secretions of animals immunized through an intestinal fistula. IgM and IgA antibody responses were low or undetectable. Low numbers of IgA and IgG1 plaque-forming cells were occasionally detected. It is proposed on the basis of these data that migration of antigen-stimulated IgG lymphoblasts, and perhaps of antigen, to spleen and peripheral lymph nodes may be dominant events after intestinal immunization of ruminants. This is consistent with the predominance of serum-derived IgG antibodies in colostrum and milk. Intramammary infusion of antigen gave rise to increases in antibody titers in all classes which were greater not only in lacteal secretions but also in blood serum than with either systemic route used. There was clear evidence from relative antibody titers for local synthesis of antibodies, principally IgA and IgG1, in the immunized glands. Comparison of IgA titers in secretions from the immunized glands with those in serum also suggested that locally synthesized IgA antibodies may have contributed in some measure to serum titers. Local synthesis in both immunized and nonimmunized glands was also reflected by the presence of increased numbers of IgA and IgG1 plaque-forming cells. It is hypothesized that antibody-forming cells responsible for local synthesis originated in lymphoid tissue within the mammary gland or from peripheral lymph nodes, depending upon the route of immunization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen W. D., Porter P. Localization of immunoglobulins in intestinal mucosa and the production of secretory antibodies in response to intraluminal administration of bacterial antigens in the preruminant calf. Clin Exp Immunol. 1975 Sep;21(3):407–418. [PMC free article] [PubMed] [Google Scholar]
- Bohl E. H., Gupta R. K., Olquin M. V., Saif L. J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972 Sep;6(3):289–301. doi: 10.1128/iai.6.3.289-301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. E., Maxwell C. F., Pierce C. S., Hylton M. B., Asofsky R., Kiddy C. A. Studies on the relative synthesis and distribution of IgA and IgG1 in various tissues and body fluids of the cow. J Immunol. 1972 Jul;109(1):38–46. [PubMed] [Google Scholar]
- Crago S. S., Prince S. J., Pretlow T. G., McGhee J. R., Mestecky J. Human colostral cells. I. Separation and characterization. Clin Exp Immunol. 1979 Dec;38(3):585–597. [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Rabbit Peyer's patches, appendix, and popliteal lymph node B lymphocytes: a comparative analysis of their membrane immunoglobulin components and plasma cell precursor potential. J Immunol. 1975 Jan;114(1 Pt 2):492–502. [PubMed] [Google Scholar]
- Curtain C. C., Clark B. L., Dufty J. H. The origins of the immunoglobulins in the mucous secretions of cattle. Clin Exp Immunol. 1971 Feb;8(2):335–344. [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Bourne F. J. Immunoglobulin quantitation in sow serum, colostrum and milk and the serum of young pigs. Biochim Biophys Acta. 1971 Apr 27;236(1):319–332. doi: 10.1016/0005-2795(71)90181-4. [DOI] [PubMed] [Google Scholar]
- Duncan J. R., Wilkie B. N., Hiestand F., Winter A. J. The serum and secretory immunoglobulins of cattle: characterization and quantitation. J Immunol. 1972 Apr;108(4):965–976. [PubMed] [Google Scholar]
- Fey H., Pfister H., Messerli J., Sturzenegger N., Grolimund F. Methods of isolation, purification and quantitation of bovine immunoglobulins: a technical review. Zentralbl Veterinarmed B. 1976 May;23(4):269–300. doi: 10.1111/j.1439-0450.1976.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Harrington J. C., Fenton J. W., 2nd, Pert J. H. Polymer-induced precipitation of antigen-antibody complexes: "precipiplex" reactions. Immunochemistry. 1971 May;8(5):413–421. doi: 10.1016/0019-2791(71)90504-0. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles A. K., McDowell G. H. Localized humoral immunity with particular reference to ruminants. Transplant Rev. 1974;19(0):170–208. doi: 10.1111/j.1600-065x.1974.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Lascelles A. K. The histological changes in involuting mammary glands of ewes in relation to the local allergic response. Aust J Exp Biol Med Sci. 1969 Oct;47(5):613–623. doi: 10.1038/icb.1969.155. [DOI] [PubMed] [Google Scholar]
- Mach J. P., Pahud J. J. Secretory IgA, a major immunoglobulin in most bovine external secretions. J Immunol. 1971 Feb;106(2):552–563. [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- McDowell G. H., Lascelles A. K. Local production of antibody by the lactating mammary gland of the ewe and the effect of systemic immunization. Res Vet Sci. 1971 Mar;12(2):113–118. [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B. Passive immunity in the foal: measurement of immunoglobulin classes and specific antibody. Am J Vet Res. 1973 Oct;34(10):1299–1303. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Invest. 1978 Mar;61(3):731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Mestecky J., Arnold R. R., Bozzo L. Ingestion of Streptococcus mutans induces secretory immunoglobulin A and caries immunity. Science. 1976 Jun 18;192(4245):1238–1240. doi: 10.1126/science.1273589. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Rosner B. R., Cohn J. The secretory antibody response. Anti-DNP antibodies induced by dinitrophenylated type 3 pneumococcus. Immunol Commun. 1974;3(2):143–156. doi: 10.3109/08820137409055752. [DOI] [PubMed] [Google Scholar]
- Newby T. J., Bourne F. J. The nature of the local immune system of the bovine small intestine. Immunology. 1976 Sep;31(3):475–480. [PMC free article] [PubMed] [Google Scholar]
- Newby T. J., Bourne J. The nature of the local immune system of the bovine mammary gland. J Immunol. 1977 Feb;118(2):461–465. [PubMed] [Google Scholar]
- Norcross N. L. Immune response of the mammary gland and role of immunization in mastitis control. J Am Vet Med Assoc. 1977 May 15;170(10 Pt 2):1228–1233. [PubMed] [Google Scholar]
- Porter P., Noakes D. E., Allen W. D. Intestinal secretion of immunoglobulins in the preruminant calf. Immunology. 1972 Sep;23(3):299–312. [PMC free article] [PubMed] [Google Scholar]
- Porter P., Noakes D. E., Allen W. D. Secretory IgA and antibodies to Escherichia coli in porcine colostrum and milk and their significance in the alimentary tract of the young pig. Immunology. 1970 Feb;18(2):245–257. [PMC free article] [PubMed] [Google Scholar]
- Roux M. E., McWilliams M., Phillips-Quagliata J. M., Weisz-Carrington P., Lamm M. E. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977 Nov 1;146(5):1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik O., Perey D. Y., Bienenstock J. Differential IgA repopulation after transfer of autologous and allogeneic rabbit Peyer's patch cells. J Immunol. 1975 Jan;114(1 Pt 1):40–44. [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Gupta R. K. Isolation of porcine immunoglobulins and determination of the immunoglobulin classes of transmissible gastroenteritis viral antibodies. Infect Immun. 1972 Oct;6(4):600–609. doi: 10.1128/iai.6.4.600-609.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. L., Muir L. A., Ferguson L. C., Conrad H. R. Selective transport of IgGl into the mammary gland: role of estrogen and progesterone. J Dairy Sci. 1971 Dec;54(12):1886–1894. doi: 10.3168/jds.S0022-0302(71)86130-1. [DOI] [PubMed] [Google Scholar]
- Sweet G. H., Welborn F. L. Use of chromium chloride as the coupling agent in a modified plaque assay. Cells producing anti-protein antibody. J Immunol. 1971 May;106(5):1407–1410. [PubMed] [Google Scholar]
- Watson D. L., Lascelles A. K. The influence of systemic immunisation during mammary involution on subsequent antibody production in the mammary gland. Res Vet Sci. 1975 Mar;18(2):182–185. [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]
- Yokomizo Y., Norcross N. L. Bovine antibody against Streptococcus agalactiae, type Ia, produced by preparturient intramammary and systemic vaccination. Am J Vet Res. 1978 Mar;39(3):511–516. [PubMed] [Google Scholar]


