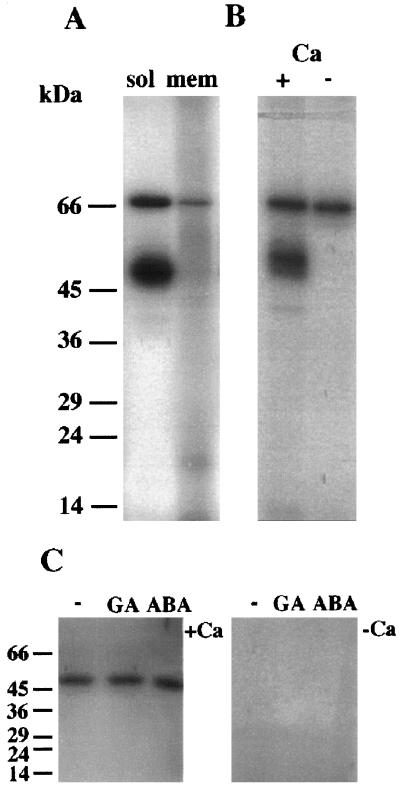
Figure 8.
Autophosphorylation characteristics of barley aleurone kinase activities. A, Autophosphorylating activities of kinases in soluble or microsomal fractions extracted from aleurone layers. Assays were carried out in the presence of 50 μm CaCl2 using 50 μg of protein per fraction. B, Autophosphorylation activities of kinases in a crude fraction were carried out in the presence of 50 μm CaCl2 (+) or 500 μm EGTA (−). C, Aleurone layers were treated for 24 h with no hormones (−), 5 μm GA, or 5 μm ABA. Crude extracts of proteins were then prepared, run on 12% SDS-PAGE, renatured, and assayed for autophosphorylating activity in gel. For these assays incubation with [32P]ATP at a concentration of 1 μm (50,000 dpm pmol−1) was carried out in the presence of 50 μm CaCl2 (+Ca) or 1 mm EGTA (−Ca). Note that only the 54-kD kinase is renatured under these conditions. The positions of molecular mass markers are indicated in kilodaltons on the left.