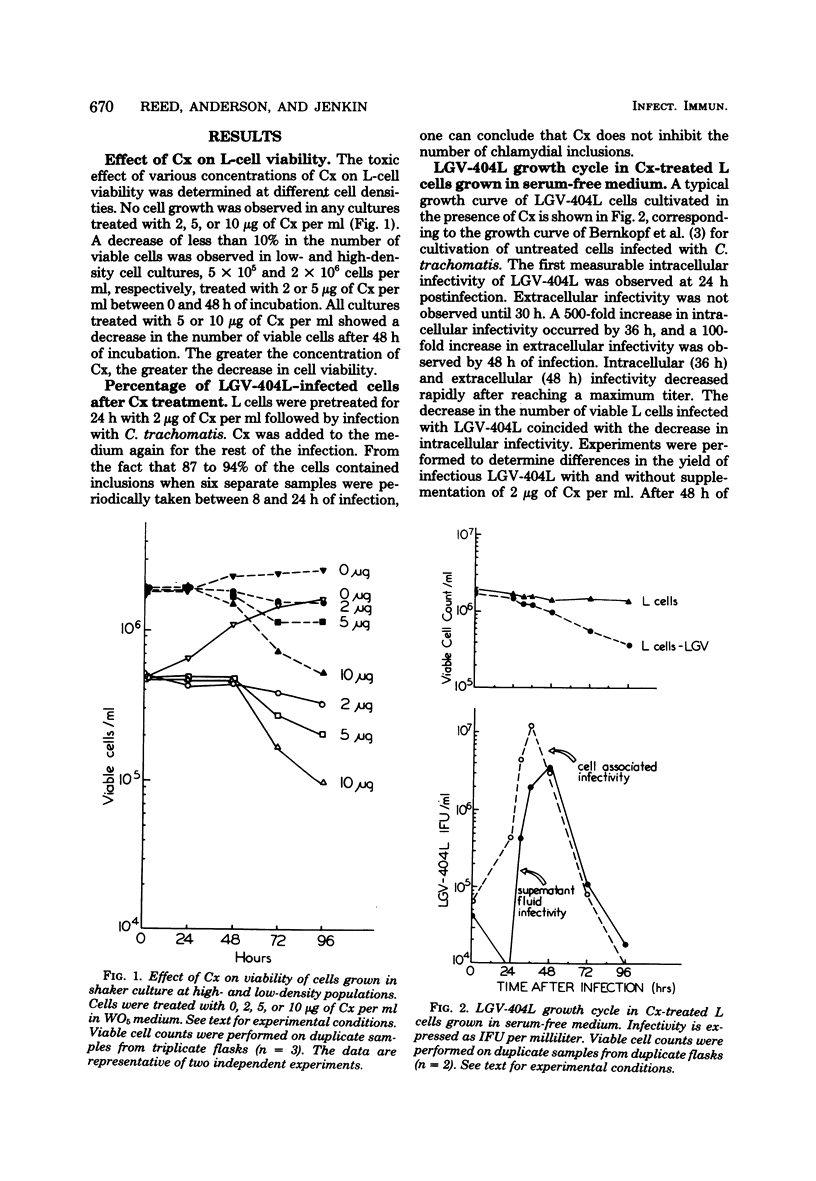
Abstract
A system for measuring chlamydial lipid synthesis was developed with mouse L cells grown in serum-free modified Waymouth 752/l medium in a shaker culture. Host lipid synthesis was reduced approximately 90% when cells were incubated for 24 h in medium containing cycloheximide (2 micrograms/ml). Lipid metabolism was monitored by measuring the incorporation of [3H]isoleucine into the total lipid of normal and infected cells. The results suggested that lipid synthesis of Chlamydia trachomatis lymphogranuloma venereum (LGV-404L) was not inhibited by cycloheximide treatment when the chlamydiae were grown in L cells, whereas host lipid synthesis was inhibited. Chlamydial lipid metabolism began about 6 to 12 h after infection when the noninfectious reticulate body was found and continually increased until the beginning of the appearance of intracellular infectious elementary bodies at 24 to 30 h.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNKOPF H., MASHIAH P., BECKER Y. Correlation between morphological and biochemical changes and the appearance of infectivity in FL cell cultures infected with trachoma agent. Ann N Y Acad Sci. 1962 Mar 5;98:62–81. doi: 10.1111/j.1749-6632.1962.tb30532.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COON M. J. Enzymatic synthesis of branched chain acids from amino acids. Fed Proc. 1955 Sep;14(3):762–764. [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. CYCLOHEXIMIDE: ASPECTS OF INHIBITION OF PROTEIN SYNTHESIS IN MAMMALIAN CELLS. Science. 1964 Dec 11;146(3650):1474–1476. doi: 10.1126/science.146.3650.1474. [DOI] [PubMed] [Google Scholar]
- Gaugler R. W., Neptune E. M., Adams G. M., Sallee T. L., Weiss E., Wilson N. N. Lipid synthesis by isolated Chlamydia psittaci. J Bacteriol. 1969 Nov;100(2):823–826. doi: 10.1128/jb.100.2.823-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskey L. E., Jenkin H. M. The serial cultivation of suspended BHK-21/13 cells in serum-free Waymouth medium. Proc Soc Exp Biol Med. 1976 Jan;151(1):221–224. doi: 10.3181/00379727-151-39178. [DOI] [PubMed] [Google Scholar]
- HULL R. N., CHERRY W. R., TRITCH O. J. Growth characteristics of monkey kidney cell strains LLC-MK1, LLC-MK2, and LLC-MK2(NCTC-3196) and their utility in virus research. J Exp Med. 1962 May 1;115:903–918. doi: 10.1084/jem.115.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol Rev. 1977 Jun;41(2):391–418. doi: 10.1128/br.41.2.391-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Wentworth B. B., Grayston J. T. Primary isolation of TRIC organisms in HeLa 229 cells treated with DEAE-dextran. J Infect Dis. 1972 Jun;125(6):665–668. doi: 10.1093/infdis/125.6.665. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makino S., Jenkin H. M., Yu H. M., Townsend D. Lipid composition of Chlamydia psittaci grown in monkey kidney cells in defined medium. J Bacteriol. 1970 Jul;103(1):62–70. doi: 10.1128/jb.103.1.62-70.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Jenkin H. M. Growth of Chlamydia psittaci strain meningopneumonitis in mouse L cells cultivated in a defined medium in spinner cultures. In Vitro. 1972 Sep-Oct;8(2):94–100. doi: 10.1007/BF02615966. [DOI] [PubMed] [Google Scholar]
- Raff R. A. Induction of fatty acid synthesis in cultured mammalian cells: effects of cycloheximide and X-rays. J Cell Physiol. 1970 Jun;75(3):341–351. doi: 10.1002/jcp.1040750310. [DOI] [PubMed] [Google Scholar]
- Ripa K. T., Mårdh P. A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J Clin Microbiol. 1977 Oct;6(4):328–331. doi: 10.1128/jcm.6.4.328-331.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Cycloheximide-resistant glycosylation in L cells infected with Chlamydia psittaci. Infect Immun. 1974 Mar;9(3):497–499. doi: 10.1128/iai.9.3.497-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


