Abstract
Epidemiological studies indicate a positive correlation between alcohol consumption and the risk of developing breast cancer. However, little is known about whether alcohol consumption affects breast cancer metastasis. Considering the primary cause of death in breast cancer patients is due to metastasis, further insight into whether alcohol consumption influences disease progression and survival is needed. We tested the effect of alcohol consumption on breast cancer metastasis using the 4T1.2 syngeneic mammary tumor model in Balb/c mice. The treatment groups included a High-consuming group (18% w/v alcohol in drinking water), a Moderate-consuming group (5% w/v), a Low-consuming group (1% w/v), and a Water-drinking control group. 4T1.2 mammary tumor cells were injected orthotopically into the mammary fat pad. Metastases were enumerated in lungs and in distant mammary glands four weeks after injection. Consumption of High alcohol protected against metastasis, as High-consuming mice typically had 65 – 75% fewer metastases compared to Water-drinking controls. A suggestive reduction in tumor spread was observed in the Moderate-drinking group, although the effects did not reach statistical significance. Consumption of the Low alcohol dose did not affect metastasis. CXCR4 expression in the primary tumors was significantly reduced by High alcohol consumption; however, expression of this chemokine receptor in the primary tumor did not correlate with metastatic potential. Additional studies were conducted to test for possible direct effects of 0.3% w/v ethanol on tumor cell proliferation, migration, invasion, and colony formation of 4T1.2 cells in vitro. Our results indicate that, for this murine model, alcohol consumption does not exacerbate tumor metastasis, and that high alcohol consumption reduces tumor spread.
Keywords: Breast cancer, Metastasis, Ethanol, Alcohol, CXCR4, Mice
Introduction
Breast cancer is diagnosed in over a quarter million women in the US each year, and it is currently the second leading cause of cancer deaths [1]. Advancing age, heritable mutations in tumor suppressor genes, and family history of breast cancer are among the strongest risk factors identified to date; however, numerous environmental, hormonal, reproductive, and dietary factors also are likely to influence the risk of disease. Consumption of alcohol is one dietary factor generally recognized to increase the risk of breast cancer, particularly for women who drink heavily [2–4]. The magnitude of risk from alcohol is likely influenced by numerous factors in addition to the amount, including the pattern of consumption, obesity, hormonal status, and ethnic background [5, 6]. Due to the evidence linking alcohol consumption with breast and other cancers, The American Institute for Cancer Research currently recommends that women who consume alcohol limit their exposure to 1 drink per day [4].
Currently there are over 2.6 million women living in the US with a history of breast cancer [1]. Given that many women living with breast cancer consume alcohol, it is important to know whether continued alcohol use has an impact on the course of the disease. Epidemiologic analyses of patients with breast cancer provide some insight into the effect of drinking on cancer recurrence and mortality; however, the number of studies specifically addressing these outcomes is limited. Existing evidence suggests that alcohol consumption does not negatively influence all-cause mortality, and may even improve life expectancy, possibly via improved cardiovascular health [7–9]. For breast cancer-specific mortality or recurrence, a small number of studies report that alcohol use increases recurrence, metastasis, or mortality, while other studies fail to find any association between post-diagnosis alcohol use and disease progression [7–10]. Little is known regarding the effect of alcohol consumption on breast cancer metastasis.
In the current studies we tested the effect of Low to High chronic alcohol consumption on breast cancer metastasis using a well-established murine model. Mice were given 1%, 5% or 18% w/v ethanol in the drinking water for a minimum of 6 weeks prior to tumor injection and throughout the tumor growth phase. Syngeneic 4T1.2 tumor cells were injected into the mammary gland fat pad of female Balb/c mice, and primary tumor growth and metastasis were evaluated during the following 4 weeks. The 4T1.2 subline of 4T1 tumor cells, originally derived from a spontaneous mammary carcinoma in a Balb/c mouse [11], spontaneously metastasizes in a pattern analogous to human breast cancer, and is one of the best available models for breast cancer metastasis [12, 13].
Materials and Methods
Animals and alcohol administration
Female immunocompetent Balb/c mice were purchased from NCI-Charles River (Waltham, MA) at 6 weeks of age. Animals were housed singly in microioslator cages, given food and water/alcohol ad libitum, and maintained on a 12:12 hour light cycle. Alcohol was given via drinking water at 3 different weight/volume (w/v) ethanol concentrations: “High” = 18%, “Moderate” = 5%, and “Low” = 1%. Alcohol was prepared by diluting Everclear™ in sterile deionized water. The High alcohol-consuming mice were gradually phased up from 5% w/v ethanol to 18% w/v ethanol over the first 2 weeks. Mice were exposed to alcohol for a total of 6–8 weeks prior to tumor cell injection, and maintained on alcohol after tumor injection for the duration of the study. Water-drinking mice were included as controls. In Experiments 2 & 3, some of the Water-drinking mice received weekly oral exposures of ~200 µl peanut oil, because they also served as vehicle controls for an unrelated chemical treatment. Statistical analysis revealed no effect of peanut oil on tumor metastasis compared to Water alone. Liquid consumption was monitored throughout the experiment.
4T1.2 tumor model
The highly metastatic 4T1.2 breast cancer cell line [11] was provided by Cheryl Jorcyk (Boise State University, Boise ID), and used with permission of Robin Anderson (Peter MacCallum Cancer Centre, East Melbourne, Australia). Cells were routinely cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, sodium pyruvate, and antibiotics. Balb/c mice were anesthetized with ketamine and xylazine, and a small incision was made in the skin to expose the right abdominal mammary gland fat pad. 4T1.2 cells (1×105 in 50 µl PBS) were injected into the fat pad, and the wound was closed with wound clips. Liquid consumption was measured weekly throughout the study. Mice were euthanized between 3.5 and 4 weeks after tumor cell injection. This time frame was selected because lung tumor burden is high, and because the mice typically begin to show signs of morbidity during weeks 3 to 4. The number of animals in each treatment group was 10–20, depending on the study.
Evaluation of primary tumors and metastases
Mice were euthanized 3.5 – 4 weeks after injection of 4T1.2 cells, depending on the experiment. Primary tumors were excised and weighed. Lungs were fixed in Bouin’s solution, and metastases were quantified by counting using a dissecting microscope. Tumor nodules that had formed on the mammary glands distant from the primary tumor (right and left thoracic and cervical glands, and the left abdominal gland) were also counted at necropsy [14]. No overt macro-metastases to organs other than the lung and distant mammary glands were consistently observed.
Morbidity
The condition of the individual animals was evaluated, using a scoring rubric, with regard to appearance, body condition, natural behavior, and provoked behavior. A value of 1–3 was assigned for each of these 4 categories. A total score of 12 indicates normal/healthy, lower scores indicate progressive morbidity, and a score of 5 was an automatic endpoint.
Cell proliferation assay
4T1.2 cells were cultured in 96-well plates in media containing 0.1%, 0.2%, or 0.3% w/v ethanol. Media were changed daily. Cell proliferation was measured on days 1 – 4 using WST-1 Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA).
Colony formation assay
4T1.2 cells were pre-treated with 0.3% w/v ethanol for 48 hours prior to suspension in agar. A suspension of cells was prepared in 0.35% agar and added to a 6-well plate (103 cells per well) over a 0.5% base agar layer. 0.3% w/v ethanol was present in both the top and base agar layers. Control cultures were treated similarly but without addition of ethanol. The agar suspensions were incubated at 37°C in a humidified incubator for 3 weeks. At termination, the colonies were stained with crystal violet and counted on a dissecting microscope. Positive colonies were defined as clusters consisting of more than 30 cells.
Migration and invasion assays
4T1.2 cells were pre-treated with 0.3% w/v ethanol for 24 hours. The cells were then trypsinized and resuspended in FBS-free media containing 0.1% BSA and 0.3% w/v ethanol. Control cultures were treated similarly but without addition of ethanol. Cell suspensions were added to the top chambers of a transwell insert (for migration assay) or Matrigel-coated transwell insert (for invasion assay) containing an 8 µm pore size membrane (BD Biosciences). The bottom chamber contained RPMI-1640 medium containing 10% FBS as a chemoattractant. The medium for the treated cultures also contained 0.3% w/v ethanol. Cells were incubated for 24 hrs at 37°C, at which time cells remaining in the insert were removed with a cotton swab. Migrated or invaded cells on the bottom of the filters were stained with crystal violet and counted under a microscope. The number of invaded/migrated cells is represented as the total count from 5–9 fields of view, depending on the experiment.
Western Blotting
Proteins from primary tumors (60 µg per sample), and from 4T1.2 cells exposed to 0.3% w/v ethanol for 48 hours (40 µg per sample), were subjected to SDS-PAGE as described previously [15]. Blots were probed with antibodies against CXCR4 (Abcam, Cambridge, MA) and actin (Santa Cruz Biotechnology, Santa Cruz, CA), followed by goat anti-rabbit and rabbit anti-goat HRP-conjugated secondary antibodies (Dako Cytomation, Carpinteria, CA). Bands were visualized and quantified using ChemiDoc imaging system and Image Lab software (Bio-Rad, Hercules, CA).
Statistics
Tumor metastasis data were compared using non-parametric analyses, due to non-normal distribution of data. A Mann Whitney test was used for analysis of experiments with only 2 treatment groups. For analyzing experiments with 3 or more treatment groups, a Kruskal-Wallis test was performed, followed by post-hoc Dunn’s Multiple Comparison of each alcohol group to Water-drinking controls. For body, tumor and spleen weight data, a Student’s t-test was used for experiments with only 2 treatment groups. For experiments with 3 or more treatment groups, a one-way ANOVA was performed, followed by post-hoc Dunnett’s test comparing each alcohol group to Water-drinking controls. Pearson correlation was used to test for a relationship between CXCR4 expression and lung metastases. Statistical analyses were conducted using GraphPad Prism (GraphPad Software Inc.), and a p value of ≤ 0.05 was considered statistically significant.
RESULTS
Effect of alcohol consumption on tumor metastasis
Three experiments were conducted to test whether Low, Moderate, and High alcohol consumption affected metastasis of breast cancer. The average daily consumption of ethanol for mice in each treatment group is shown in Table 1.
Table 1.
Average daily intake of ethanol
| Low alcohol | Moderate alcohol |
High alcohol | |
|---|---|---|---|
| Prior to tumor injection | 1.6 (0.05) | 7.9 (0.4) | 26.2 (1.7) |
| After tumor injection | 1.6 (0.05) | 8.7 (0.5) | 26.8 (1.2) |
Data represent the mean (±SEM) intake of alcohol per day (g ethanol per kg body weight) for all animals within the treatment group. Data are from Experiment 1.
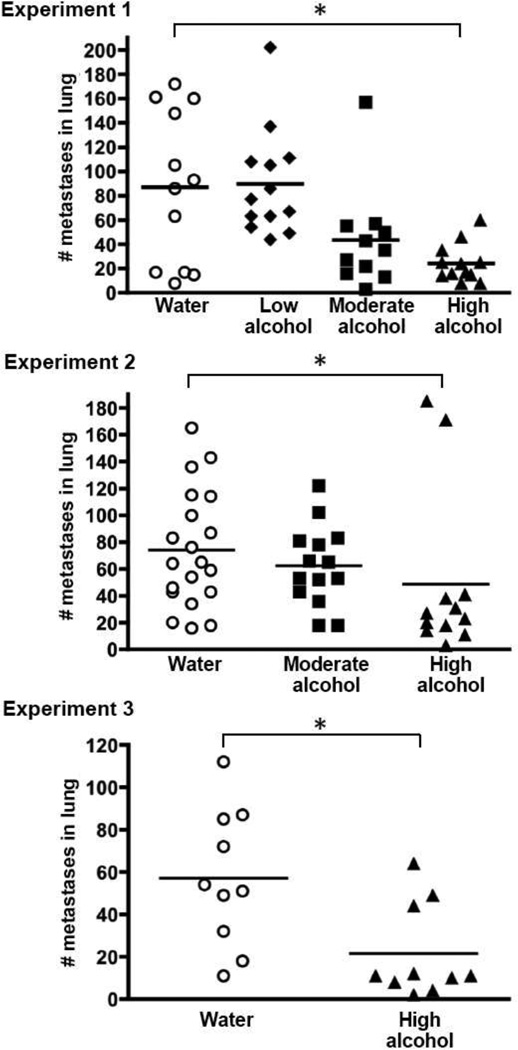
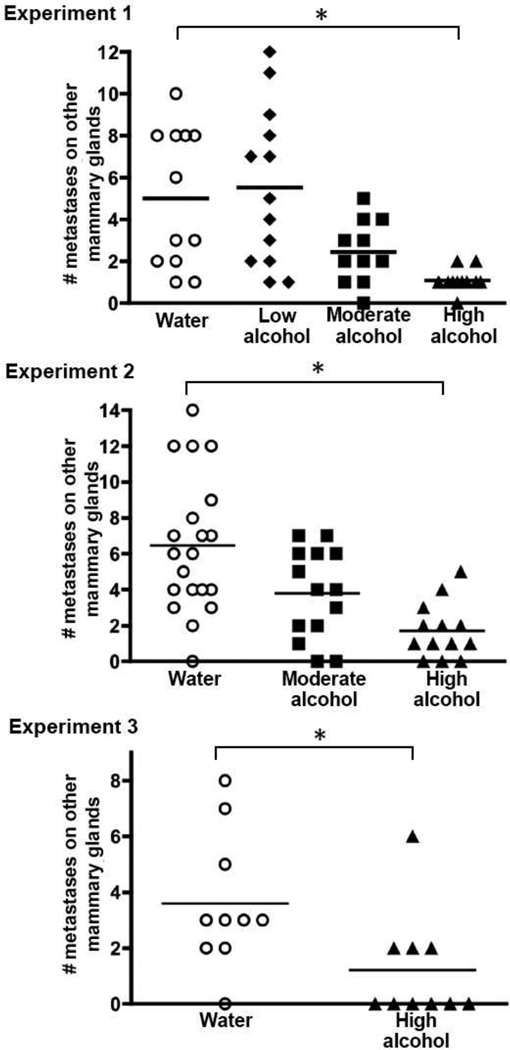
Intriguingly, consumption of High levels of alcohol significantly suppressed metastasis, and the degree of suppression was typically 65–75%. Figure 1 shows the number of 4T1.2 tumors that spread to the lungs, which was suppressed in the High-consuming mice in all 3 experiments. Metastasis to the distant mammary glands was similarly reduced, both in terms of the total number of tumor metastases that developed in each animal (Fig. 2), and in the number of distant gland sites that were tumor-positive (Table 2). For example, 50% of the Water-drinking mice in Experiment 1 had metastases in all 3 of the distant gland sites evaluated, whereas none of the High-consuming mice had tumors at all 3 sites (Table 2).
Fig. 1.
Effect of drinking alcohol on mammary tumor metastasis to the lung. Three separate experiments were conducted using Balb/c mice injected with 4T1.2 tumor cells in the fat pad of the right abdominal mammary gland. Alcohol was provided in drinking water at indicated w/v concentrations: “High” = 18%, “Moderate” = 5%, and “Low” = 1%. Mice were exposed to alcohol for 6 weeks prior to tumor cell injection, and throughout the 4-week tumor growth phase. At termination, lungs were removed and fixed in Bouin’s solution to facilitate counting tumor colonies. Symbols represent the number of visible metastases for individual mice in each treatment group. Experiment #1 was conducted with groups of mice at High, Moderate, and Low alcohol concentrations and Water-drinking controls (n = 11–13 per group); Experiment #2 included High and Moderate groups and Water-drinking controls (n = 13–20 per group); and Experiment #3 compared the High concentration group to Water controls (n=10 mice per group). An * indicates p ≤ 0.05
Fig. 2.
Tumor metastasis to distant mammary glands is suppressed by alcohol consumption. Mice were treated as described in Fig. 1. Tumor nodules on mammary glands at 3 sites distant from the primary tumor site (the right thoracic and cervical glands, the left thoracic and cervical glands, and the left abdominal gland sites) were counted at necropsy. Symbols represent the number of visible metastases for individual mice in each treatment group. An * indicates p ≤ 0.05
Table 2.
Percentage of mice with tumors at distant mammary gland sites
| % of mice with tumors at distant gland sites | |||||
|---|---|---|---|---|---|
| 0 sites | 1 site | 2 sites | 3 sites | ||
| Experiment 1 | Water | 0 | 17 | 33 | 50 |
| Low alcohol | 0 | 15 | 39 | 46 | |
| Moderate alcohol | 9 | 27 | 46 | 18 | |
| High alcohol | 8 | 75 | 17 | 0 | |
| Experiment 2 | Water | 5 | 5 | 10 | 80 |
| Moderate alcohol | 14 | 7 | 22 | 57 | |
| High alcohol | 23 | 31 | 23 | 23 | |
| Experiment 3 | Water | 10 | 10 | 40 | 40 |
| High alcohol | 60 | 10 | 20 | 10 | |
Tumor spread from the primary site (right abdominal mammary gland) to any of 3 distant gland sites (the left abdominal gland, the right thoracic and cervical glands, and the left thoracic and cervical glands) was determined at necropsy. Tabulated data are the percentage of animals that had tumors at 0, 1, 2, or all 3 of the distant sites.
Results for the Moderate alcohol group revealed a trend toward suppression of tumor metastasis, although the effects did not reach statistical significance at p ≤ 0.05. In particular, Moderate alcohol consumption suggestively diminished the number of metastases in the distant mammary glands in both Experiments 1 and 2 (Fig. 2), and fewer Moderate-drinking mice had tumors at all three gland sites compared to Water-drinking controls (Table 2). With regard to metastases enumerated in the lung, the results were inconsistent for the Moderate-consuming group. There was a suggestive suppression in pulmonary metastases in Experiment 1, but no evidence for an effect in Experiment 2 (Fig. 1).
There was no apparent effect of the Low concentration of alcohol on tumor spread, either to the lung or to the distant mammary glands (Experiment 1, in Figs. 1 and 2 and Table 2).
Alcohol consumption did not effect the establishment of 4T1.2 tumors after implantation into the mammary fat pad
One plausible explanation for the observed effect on metastasis was that alcohol consumption interfered with growth of the primary tumor at the site of injection. To address this possibility, tumor growth in the implanted mammary gland was monitored weekly by external measurements (not shown), and the weight of the primary tumor was recorded at the time of necropsy (Table 3). We found no evidence that consumption of alcohol delayed tumor onset or decreased primary tumor growth.
Table 3.
Weight of primary tumor is unaffected by alcohol
| Water | Low alcohol |
Moderate alcohol |
High alcohol |
|
|---|---|---|---|---|
| Experiment 1 | 790a (70) | 850 (70) | 700 (10) | 700 (10) |
| Experiment 2 | 700 (30) | - | 800 (60) | 740 (50) |
| Experiment 3 | 960 (90) | - | - | 820 (120) |
Mean weight in mg (± SEM) of tumors excised from the primary site at the termination of each study.
‘-‘ indicates the treatment group was not included in that study.
Effect of alcohol on body weight and morbidity
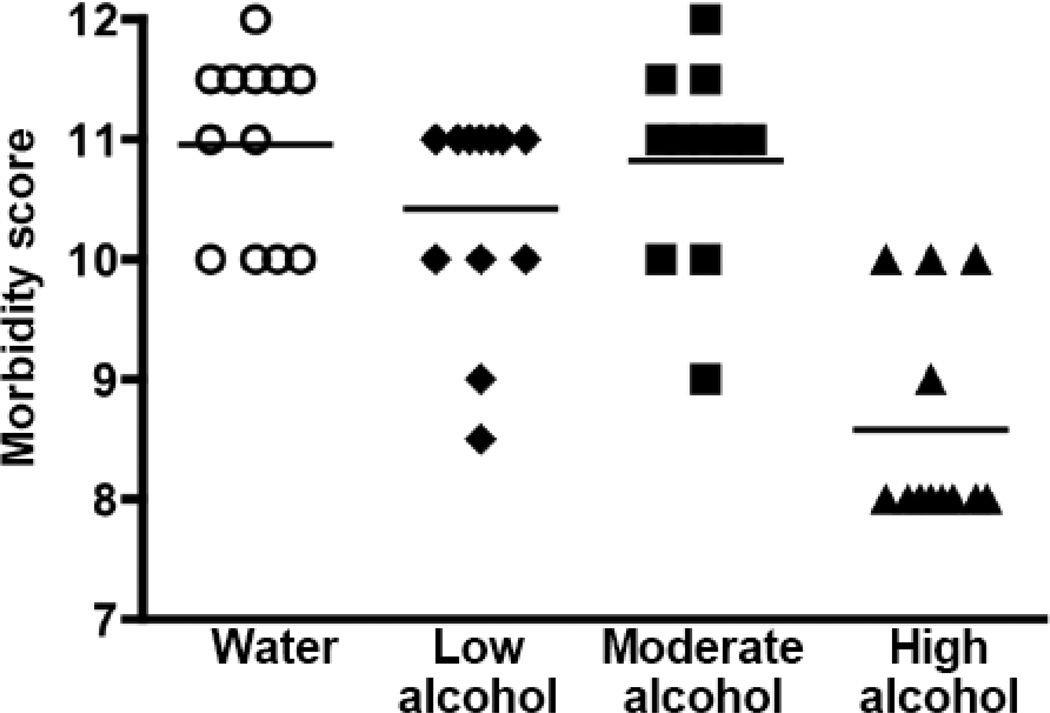
Despite the fact that High alcohol consumption reduced tumor metastasis, there was no evidence that this was associated with improvement in the overall health of the animals. In fact, two pieces of evidence suggested the alcohol-drinking mice were less robust. First, the terminal body weight of the mice in the High alcohol group was typically 8–15% less than Water-drinking controls (Table 4). Second, for Experiment 1 the health of the alcohol-consuming mice declined more rapidly than Water-drinking controls as determined by a body score rubric (Fig. 3). In this particular experiment the declining health of the alcohol-drinking mice triggered early termination of the study.
Table 4.
Body weight at termination of the study
| Water | Low alcohol |
Moderate alcohol |
High alcohol |
|
|---|---|---|---|---|
| Experiment 1 | 24.4 a (0.4) | 23.3 (0.5) | 24.2 (0.3) | 20.9 (0.4) * |
| Experiment 2 | 22.0 (0.4) | - | 22.0 (0.4) | 19.3 (0.4) * |
| No tumor controlsb | 22.3 (1.5) | - | 22.7 (0.4) | 19.8 (0.9) |
| Experiment 3 | 21.1 (0.4) | - | - | 19.5 (0.8) * |
Body weight in g at necropsy, minus the weight of the primary tumor (±SEM),
Body weight of control animals (n=3–4 per group) that were not injected with tumor cells are shown for Experiment 2.
indicates significant difference from Water-drinking controls.
‘-‘ indicates the treatment group was not included in that study.
Note. The starting weight of mice was not significantly different among the different experimental groups, and mice in all treatment groups gained weight during the period before tumor injection. However, mice consuming high alcohol generally gained less weight than the other experimental groups, both for tumor-bearing mice and for mice not injected with tumor.
Fig. 3.
Effect of alcohol consumption on morbidity. Body condition was given a numerical value using a scoring rubric. Scores decreased over the final 5 days of the study, particularly in the High alcohol group. Data show the scores for individual mice on the day of euthanasia for Experiment 1
Effect of alcohol on the spleen
Splenomegaly is common in mice bearing 4T1 tumors [16], and in the current studies the average spleen weight was approximately 6-fold higher in tumor-injected animals than in “no tumor” controls (Table 5). Consumption of the High alcohol mitigated splenomegaly somewhat, and spleen weight was typically 20 – 30% lower in these animals when compared to Water-drinking controls. However, High alcohol consumption also decreased the baseline spleen weights to a similar extent in the control mice not injected with 4T1.2 cells.
Table 5.
Tumor-associated splenomegaly
| Water | Low alcohol |
Moderate alcohol |
High Alcohol |
|
|---|---|---|---|---|
| Experiment 1 | 565 a (35) | 608 (25) | 555 (36) | 413 (30) * |
| Experiment 2 | 582 (25) | - | 607 (27) | 480 (39) * |
| No tumor controlsb | 104 (4) | - | 107 (3) | 71 (7) * |
| Experiment 3 | 591 (28) | - | - | 407 (34) * |
Mean spleen weight in mg (± SEM) at the termination of each study.
Spleens from control animals (n=3–4 per group) that were not injected with tumor cells are shown for Experiment 2. This control group emphasizes the marked increase in spleen size that occurs in tumor-bearing mice, and also that alcohol consumption decreases spleen weight regardless of whether tumor is present.
‘-‘ indicates the treatment group was not included in that study.
indicates significant difference from Water-drinking controls. p<0.05.
In vitro assays of cellular activities consistent with metastasis
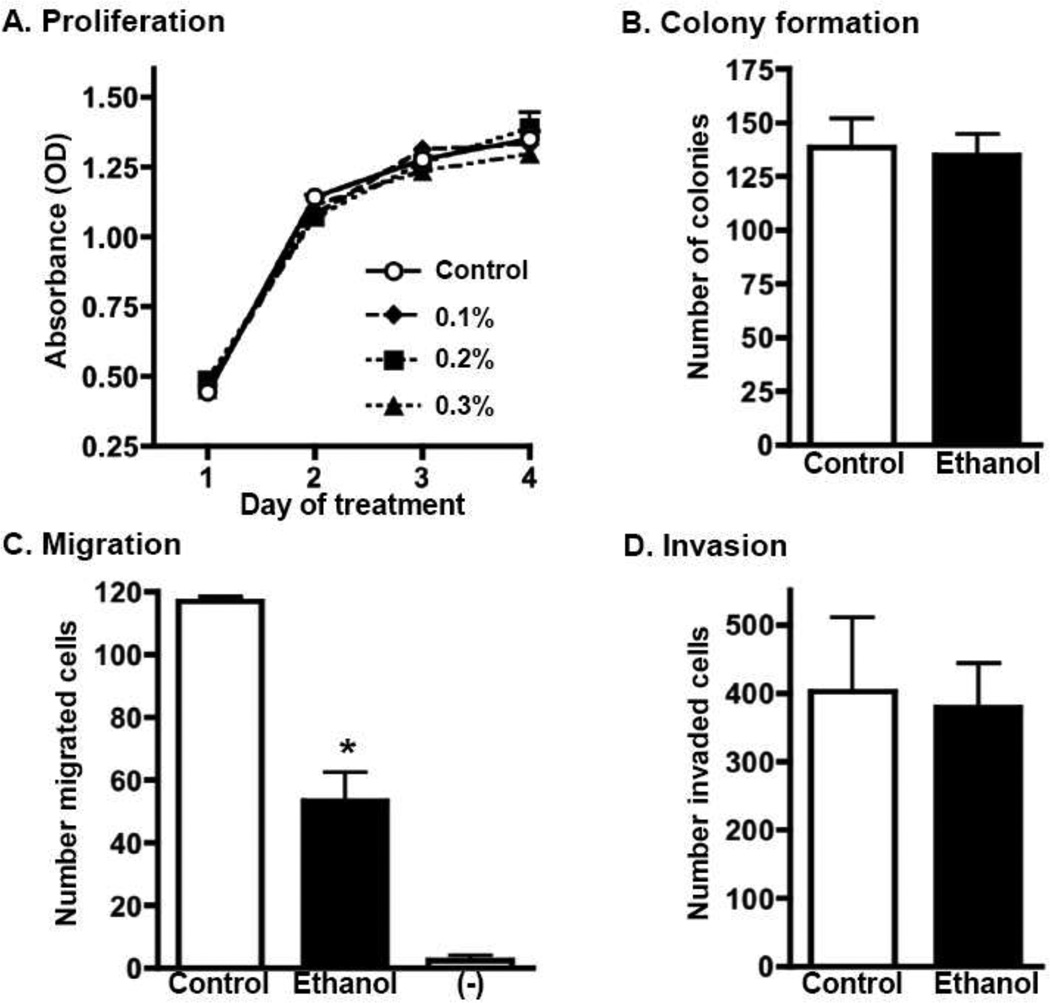
One possible explanation for the observed decrease in metastasis is that alcohol acts directly on the tumor cells to inhibit metastatic activity. To address this, we tested for direct effects of alcohol on 4T1.2 cells using in vitro assays of proliferation, migration, invasion, and colony formation (Fig. 4). Ethanol had no impact on the ability of 4T1.2 cells to proliferate (Fig. 4A), or form colonies in soft agar containing ethanol (Fig. 4B). Ethanol suppressed the ability of the tumor cells to migrate in response to FBS chemoattractant (Fig. 4C). The degree of suppression was approximately 50%, and was observed in two independent experiments. In contrast, ethanol did not affect invasion (Fig. 4D).
Fig. 4.
Effect of ethanol on in vitro assays measuring proliferation, colony formation, migration, and invasion. (A) 4T1.2 cells were treated daily with media containing indicated concentrations of ethanol. Cell proliferation was evaluated using a WST-1 assay on days 1–4 of treatment. Error bars indicate SEM of triplicate cultures. (B) 4T1.2 cells were pre-treated with 0.3% w/v ethanol for 48 hours, then cultured in semi-solid agar containing 0.3% w/v ethanol. Control cultures were not exposed to ethanol. Bar graphs show the average number of colonies that formed after 3 weeks of culture (± SEM, of triplicate cultures). (C&D) 4T1.2 cells were pre-treated with 0.3% w/v ethanol for 24 hours, then added to the top chamber of a transwell system. FBS-containing media was placed in the lower chamber as a chemoattractant. 0.3% w/v ethanol was also present in the media of both transwell chambers. Control cultures were not exposed to ethanol. “-“ indicates the amount of background cell migration in transwells containing no FBS as a chemoattractant. Bar graphs show the average number of cells (± SEM, from triplicate wells) that migrated across the semi-permeable membrane (C), or invaded through a Matrigel-coated membrane (D)
CXCR4 expression in primary tumors and cultured cells
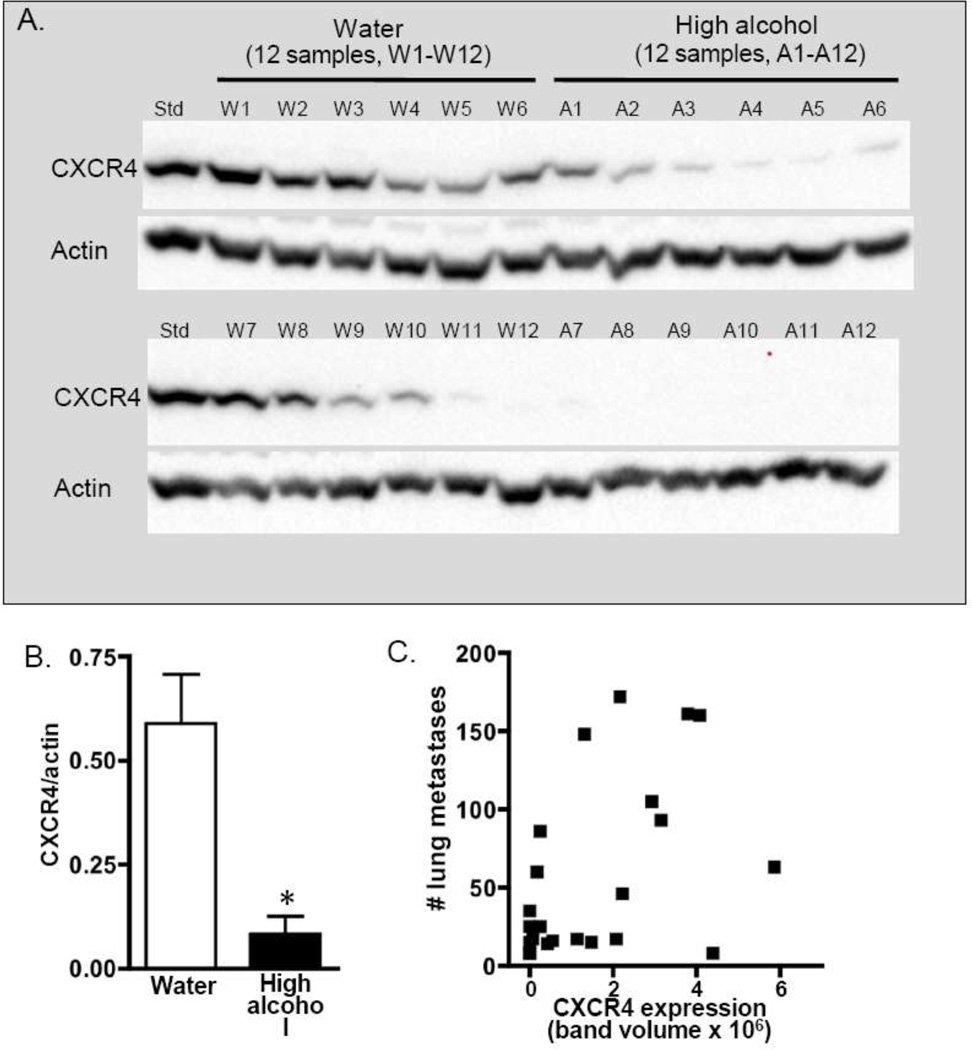
CXCR4 expression is known to influence migration of cancer cells, and we hypothesized that alcohol consumption blocks metastasis in part by suppressing this chemokine receptor. Analysis of primary tumor homogenates revealed that High alcohol-drinking mice typically had lower CXCR4 expression relative to Water-consuming animals (Fig. 5A). Overall, High alcohol consumption significantly suppressed CXCR4 expression in primary tumors by over 80% in Experiment 1 (Fig. 5B) and by approximately 60% in Experiment 2 (not shown). Next, to assess whether this diminished CXCR4 could explain the observed suppression in metastasis, we plotted CXCR4 expression level in the primary tumor against the number of pulmonary metastases for each animal. Correlation analysis revealed that there was no relationship between CXCR4 expression and metastasis in either Experiment 1 (Fig. 5C) or Experiment 2 (not shown), either when each treatment group was analyzed individually or when the groups were combined (r2=0.23 and 0.15 for Experiments 1 and 2 respectively).
Fig. 5.
Expression of CXCR4 in primary tumors. (A) CXCR4 (43 kDa) expression was examined in tumor homogenates from 12 Water-drinking and 12 High alcohol-consuming mice from Experiment 1. For presentation, samples were intentionally loaded in approximate order of band intensity, and a common sample (Std) loaded on both gels was used to ensure equivalent exposure between blots. Actin (41 kDa) was used as a loading control. (B) CXCR4 expression was quantified as band volume and normalized based on actin expression. (C) Expression of CXCR4 in each tumor sample was graphed against the number of pulmonary metastases for that animal
Finally, the potential for ethanol to directly modulate CXCR4 expression in 4T1.2 cells was addressed by exposing the cells to 0.3% w/v ethanol in culture. No suppression of CXCR4 expression was evident in either of 2 experiments (data not shown). Therefore, the ethanol-induced suppression observed in the in vitro migration assay shown in Figure 4C is not explained by perturbed expression of this chemokine receptor.
Discussion
Studies conducted in women with breast cancer generally support a link between alcohol consumption and risk of incident breast cancer; however, the effect that drinking could have on metastasis and prognosis after diagnosis is less understood. In the current studies we examined the effect of alcohol consumption on mammary tumor growth and metastasis using a syngeneic murine model of orthotopically implanted 4T1.2 cells. We observed that consumption of 18% alcohol significantly suppressed metastasis assessed at two sites (lung and distant mammary glands), whereas consumption of 1% alcohol had no effect. The intermediate concentration (5%) could be near a threshold for an effect, since the number of metastases in these mice was generally lower than in Water-drinking controls, but the effect did not reach statistical significance.
One potential explanation for the suppressed metastasis in the High alcohol-consuming mice was that ethanol directly inhibits growth of 4T1.2 tumors, however this did not turn out to be the case. Primary tumor weights were not affected by alcohol consumption in vivo, nor did ethanol directly inhibit 4T1.2 cell proliferation in vitro. We also considered the possibility that alcohol treatment would directly impair cellular processes necessary for metastasis. We examined this using in vitro assays of colony formation, migration, and invasion. The presence of ethanol had no effect on the ability of the tumor cells to form colonies in soft agar or to invade into a Matrigel matrix; however, migration across a semi-permeable membrane was significantly suppressed.
We next tested the hypothesis that alcohol consumption inhibits tumor metastasis and migration via suppressing CXCR4 expression on the tumor cells. This chemokine receptor functions in regulating cell trafficking, including migration of breast cancers [17]. For example, elevated expression of CXCR4 correlates with increased metastasis in human tumors and in 4T1 cells [18, 19]. Blocking the receptor with antagonists or RNAi decreases the ability of implanted 4T1 cells to move to the lung [17, 20]. In support of a link between alcohol consumption and decreased CXCR4, we found that more tumors from High alcohol-consuming mice had low CXCR4 expression relative to tumors from Water-drinking controls. However, interpretation of these data is complicated by the fact that the level of CXCR4 expression in the primary tumor did not correlate with the number of metastases in the lung. Additionally, CXCR4 expression in cultured 4T1.2 cells was unaffected by direct exposure to ethanol, suggesting that the alterations observed in the primary tumor resulted from indirect effects. Thus, in vivo metabolites of alcohol or other changes induced by alcohol consumption in the host mice are likely responsible for CXCR4 suppression.
Our studies revealed that consumption of High alcohol inhibited metastasis, whereas Moderate and Low alcohol consumption had marginal to no effect. Additionally, we did not find evidence that alcohol exposure had an impact on the size of the primary tumor itself. However, Wang et al [21] recently reported that alcohol consumption increased primary tumor size and the number of pulmonary nodules formed after E0771 mammary carcinoma cells were orthotopically injected in syngeneic C57BL/6 mice. There are a number of differences between the Wang et al. study and our current report that may account for the different outcomes regarding tumor growth and metastasis. In the Wang et al. study, 2% alcohol was provided for 12 hours each day, beginning 3 days prior to tumor injection and continuing through the 3-week period of tumor growth. Our study involved longer and continuous 24-hour access to alcohol before tumor injection. Thus, it is possible that the effect of alcohol consumption is influenced by the length of time the animals were exposed prior to tumor injection, as well as the amount of alcohol consumed on a daily basis. Our study was designed to model chronic drinking, in contrast to the Wang et al. study that modeled acute alcohol exposure. Chronic alcohol exposure affects many biologic processes such as hormone levels, metabolism, and the immune response, especially at high intake levels. Therefore, the physiology of the animals at the time of tumor injection would likely be different between the two exposure paradigms. This could account for the different tumor growth characteristics within the mice. Differences in metastasis of murine melanoma, wherein acute alcohol exposure enhances metastasis [22] and chronic exposure inhibits metastasis [23] supports this idea. A second possibility to explain the different outcomes between the Wang, et al study and ours relates to the estrogen receptor (ER) status of the tumor cell lines used. Specifically, alcohol consumption enhanced metastasis of the ER-positive E0771 cells used in the Wang et al. study; whereas metastasis of the ER-negative 4T1.2 tumors was either inhibited (High, 18% group) or not affected (Low, 1% group) in our studies. Alcohol consumption is known to influence estrogen-associated pathways and processes in humans and rodents, including altering hormone metabolism and increasing circulating estrogen. Thus alcohol-induced changes in estrogen levels or downstream sequelae could differentially affect growth and spread of ER-positive vs. ER–negative tumors.
To our knowledge, there is only one other rodent study that has examined the effect of alcohol on metastasis of syngeneic mammary tumors [24]. In that study, F334 male rats consumed Moderate (5%) alcohol for 2 weeks prior to and for 3 weeks following intravenous injection of MADB106 cells. Alcohol consumption was associated with a 5- fold increase in the number of tumors that formed in the lung. MADB106 cells are ER-positive, so this result supports the idea discussed above that ER-status of the tumor could influence the effect that alcohol has on tumor spread. However, since the studies were performed in males, the mechanism of the effect may be different than for the mouse studies discussed above. Further studies utilizing different tumor lines and rodent strains will be needed in order to better address the influence of species and hormone receptor status on alcohol-inhibition of metastasis. Additional alcohol exposure paradigms could also be tested. For example, women are advised to reduce their alcohol intake after breast cancer diagnosis, and this could be modeled by withdrawing alcohol from the test animals at various times during the metastasis phase.
Our results strongly indicate that alcohol consumption does not enhance metastasis in this murine model, and that High or Moderate consumption may in fact be beneficial in reducing metastasis. However, extrapolation of these findings with regard to potential implications for women with breast cancer requires cautious interpretation. One consideration is that 4T1.2 tumor cells are estrogen receptor-negative, and the effect of alcohol we observed may not translate to other tumor types. Furthermore, it is important to consider that alcohol consumption influences morbidity and mortality in numerous ways independent of tumor spread. For example, we observed that the health of the high-consuming animals declined more rapidly in at least 1 of our studies, and alcohol-consuming mice generally had reduced body weight at euthanasia. Ultimately metastasis is but one aspect of disease progression, and it is important to determine whether life expectancy or quality is affected by drinking.
Acknowledgements
The authors would like to thank Elizabeth Wright, Hiep Nguyen, Pat Ager, and Faya Zhang for technical contributions to these experiments, and Dr. Jan Dasgupta (WSU Mathematics Department) for assistance with statistical analyses.
Funding: These studies were supported by the NIH/NIAAA, including K05AA017149 to GGM with funding to BAV, R01AA07293 to GGM with a supplement to GGM and BAV, and by a grant in support of breast cancer research from the Fraternal Order of Eagles.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Contributor Information
Tao Wang, Email: taowang@vetmed.wsu.edu.
Annette K Myers, Email: annette.myers@email.wsu.edu.
Katherine L Wyrick, Email: katherine.wyrick@email.wsu.edu.
Gary G Meadows, Email: meadows@wsu.edu.
References
- 1.ACS. Breast Cancer Facts and Figures 2011–2012. Atlanta: American Cancer Society; 2011. American Cancer Society. [Google Scholar]
- 2.Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, Lessin L, O'Sullivan MJ, Wactawski-Wende J, Yasmeen S, Prentice R. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010;102:1422–1431. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelucchi C, Tramacere I, Boffetta P, Negri E, La Vecchia C. Alcohol consumption and cancer risk. Nutr Cancer. 2011;63:983–990. doi: 10.1080/01635581.2011.596642. [DOI] [PubMed] [Google Scholar]
- 4.AICR. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: American Institute for Cancer Research; 2007. World Cancer Research Fund / American Institute for Cancer Research. [Google Scholar]
- 5.Li Y, Yang H, Cao J. Association between alcohol consumption and cancers in the Chinese population--a systematic review and meta-analysis. PLoS One. 2011;6:e18776. doi: 10.1371/journal.pone.0018776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, Caan BJ. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol. 2010;28:4410–4416. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris HR, Bergkvist L, Wolk A. Alcohol intake and mortality among women with invasive breast cancer. Br J Cancer. 2012;106:592–595. doi: 10.1038/bjc.2011.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flatt SW, Thomson CA, Gold EB, Natarajan L, Rock CL, Al-Delaimy WK, Patterson RE, Saquib N, Caan BJ, Pierce JP. Low to moderate alcohol intake is not associated with increased mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:681–688. doi: 10.1158/1055-9965.EPI-09-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beasley JM, Newcomb PA, Trentham-Dietz A, Hampton JM, Bersch AJ, Passarelli MN, Holick CN, Titus-Ernstoff L, Egan KM, Holmes MD, Willett WC. Post-diagnosis dietary factors and survival after invasive breast cancer. Breast Cancer Res Treat. 2011;128:229–236. doi: 10.1007/s10549-010-1323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, Lowen D, Javni J, Miller FR, Slavin J, Anderson RL. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis. 1999;17:163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 12.Pulaski BA, Ostrand-Rosenberg S. Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, Coico R, editors. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. 2000:20.22.21–20.22.16. [Google Scholar]
- 13.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Wyrick KL, Meadows GG, Wills TB, Vorderstrasse BA. Activation of the aryl hydrocarbon receptor by TCDD inhibits mammary tumor metastasis in a syngeneic mouse model of breast cancer. Toxicol Sci. 2011;124:291–298. doi: 10.1093/toxsci/kfr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Gavin HM, Arlt VM, Lawrence BP, Fenton SE, Medina D, Vorderstrasse BA. Aryl hydrocarbon receptor activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. IntJCancer. 2011;128:1509–1523. doi: 10.1002/ijc.25493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.duPre SA, Hunter KW., Jr Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp. Mol. Pathol. 2007;82:12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Williams SA, Harata-Lee Y, Comerford I, Anderson RL, Smyth MJ, McColl SR. Multiple functions of CXCL12 in a syngeneic model of breast cancer. Mol Cancer. 2010;9:250. doi: 10.1186/1476-4598-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The elevated level of CXCR4 is correlated with nodal metastasis of human breast cancer. Breast. 2005;14:360–367. doi: 10.1016/j.breast.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Liao S, Huang Y, Samuel R, Shi T, Naxerova K, Huang P, Kamoun W, Jain RK, Fukumura D, Xu L. PDGF-D improves drug delivery and efficacy via vascular normalization, but promotes lymphatic metastasis by activating CXCR4 in breast cancer. Clin Cancer Res. 2011;17:3638–3648. doi: 10.1158/1078-0432.CCR-10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Xu M, Li F, Wang X, Bower KA, Frank JA, Lu Y, Chen G, Zhang Z, Ke Z, Shi X, Luo J. Ethanol promotes mammary tumor growth and angiogenesis: the involvement of chemoattractant factor MCP-1. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu WJ, Pruett SB. Ethanol decreases host resistance to pulmonary metastases in a mouse model: role of natural killer cells and the ethanol-induced stress response. Int J Cancer. 1999;82:886–892. doi: 10.1002/(sici)1097-0215(19990909)82:6<886::aid-ijc19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Meadows GG, Elstad CA, Blank SE, Gallucci RM, Pfister LJ. Alcohol consumption suppresses metastasis of B16-BL6 melanoma in mice. Clin Exp Metastasis. 1993;11:191–199. doi: 10.1007/BF00114977. [DOI] [PubMed] [Google Scholar]
- 24.Yirmiya R, Ben-Eliyahu S, Gale RP, Shavit Y, Liebeskind JC, Taylor AN. Ethanol increases tumor progression in rats: possible involvement of natural killer cells. Brain Behav Immun. 1992;6:74–86. doi: 10.1016/0889-1591(92)90061-r. [DOI] [PubMed] [Google Scholar]