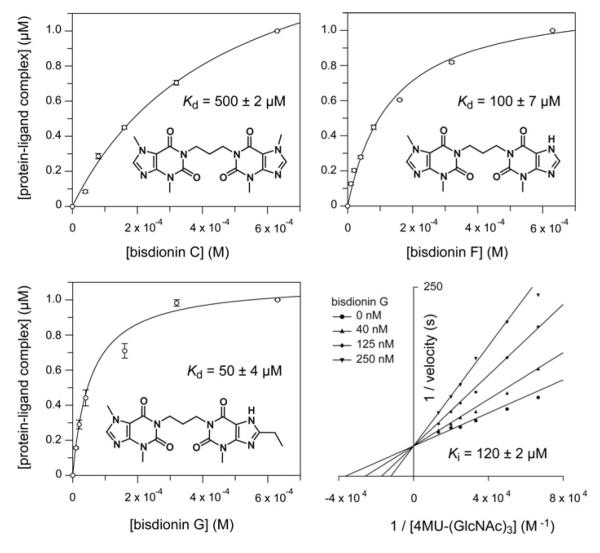
Figure 4. The bisdionins and their affinity for YKL-39.
The chemical structures of the bisdionin family of chitinase inhibitors are shown, together with bisdionin-induced changes in YKL-39 intrinsic tryptophan fluorescence fitted to a single-site-binding isotherm. Results are means ± S.D. for three independent experiments. The lower right-hand panel shows inhibition of the active mutant of YKL-39, S143D/I145E, by bisdionin G. A double-reciprocal plot is shown to visualize the competitive nature of the inhibition. The Ki value was determined by non-linear regression as described in the Materials and methods section.