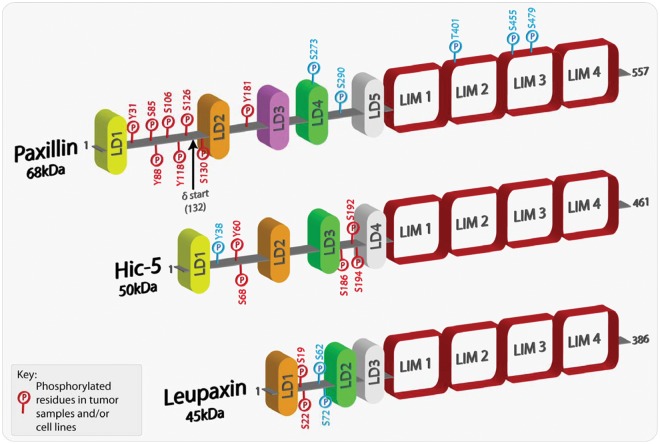
Figure 1.
Schematic representation of paxillin family members highlighting phosphorylated residues. Paxillin and its family members comprise a variable number of amino-terminal LD domains, which are short leucine-rich motifs that are responsible for mediating protein-protein interactions. At the carboxyl-terminus, each family member contains 4 highly homologous LIM domains, which also serve as protein-protein binding moieties as well as being responsible for appropriate protein subcellular localization, including sites of integrin-mediated adhesion and the nucleus. Paxillin family members can also be phosphorylated at multiple sites, with the majority located in the less conserved regions between the LD domains. Furthermore, a number of the phosphorylated residues have been revealed as mediating and regulating paxillin family member protein interactions. The phospho-residues highlighted in red have been shown in multiple studies to be phosphorylated in both tumor samples and/or cancer cell lines. The amino acids highlighted in blue have been shown to be phosphorylated in a variety of studies but thus far have not been identified in cancer cells or tissues.