Figure 3.
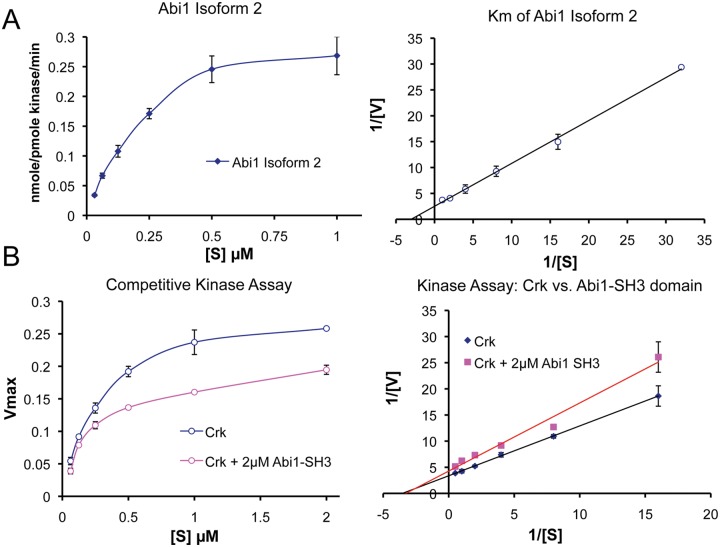
Abi1 competes with Crk at the SH3 domain interaction site. (A) Determination of Abi1 Km for Abl kinases. Km is an important value that characterizes the relative substrate affinity for a given enzyme. Moreover, Km allows the comparison of different substrates for the same enzyme. Km of purified GST Abi1 isoform 292 was determined using recombinant c-Abl (47aa-end) purified from baculovirus culture treated with Gleevec (STI-571, Novartis Pharmaceuticals, East Hanover, NJ) as described53 and using reaction conditions as described by Tanis et al.94 The Km of GST Abi1 was 0.33 ± 0.5 µM, and the Km of GST Crk was 0.32 ± 0.02 µM (see below); hence, they are comparable. (B) Abi1 competes with Crk at its Abl SH3 domain binding site. For the competition assay with GST Crk, the purified Abi1 SH3 domain was cleaved from GST and used at 2 µM. Reduction of the Km from 0.32 ± 0.02 µM to 0.17 ± 0.01 µM and reduction of the Vmax by 24% indicated mixed competition. Crk associates with Abl through binding of its SH3 domain to the Abl proline-rich domain (PRD); the Abl PRD also binds Abi149 and Abi2.55 (Left) Kinase activity plots. (Right) Double reciprocal Lineweaver-Burk plots of the kinase activity data.