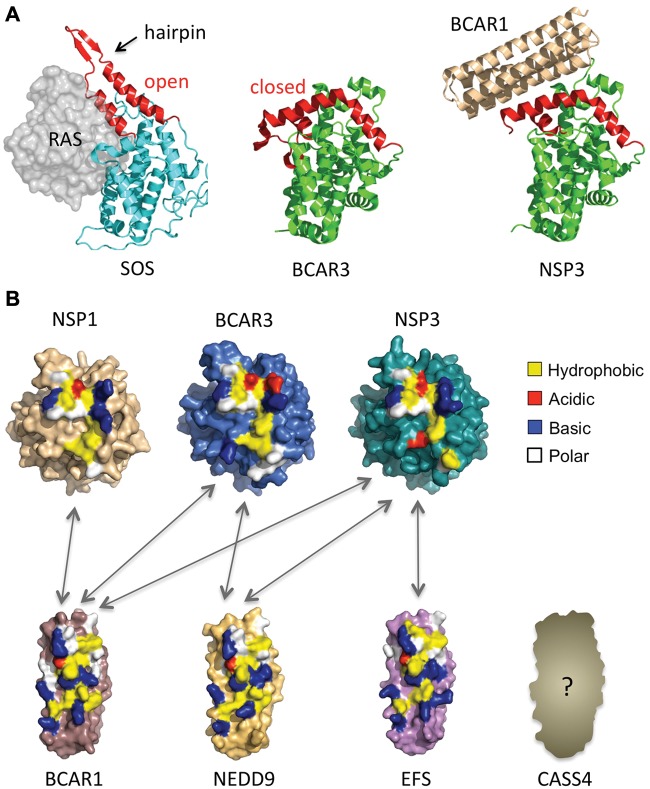
Figure 3.
Conserved interaction domains of NSP and CAS family members enable promiscuous interactions. (A) The NSP CDC25 homology domain is in a “closed” conformation that cannot bind RAS proteins but can tightly interact with CAS proteins. (Left) Structure of the CDC25 homology domain of the active exchange factor SOS bound to its target GTPase, RAS.119 (Middle) The structure of the carboxy-terminal domain of BCAR3 reveals that it also adopts a CDC25 homology domain fold, but in a new closed conformation incapable of RAS binding and exchange factor activity. (Right) The structure of the NSP3-BCAR1 complex reveals that the carboxy-terminal domain of NSP3 also adopts the closed conformation observed for BCAR3. The altered conformation enables strong interaction with the carboxy-terminal FAT domain of the CAS family protein BCAR1 to form an NSP-CAS complex, thus converting the exchange factor domain into an adaptor domain. (B) Surface representations of BCAR1, BCAR3, and NSP3 structures solved by X-ray crystallography,13 and models of NSP1, NEDD9, and EFS. The carboxy-terminus of CASS4 was not modeled due to its greater degree of sequence divergency from the structurally characterized BCAR1. Colors indicate interface residue types identified in the NSP3-BCAR1 complex and the corresponding residues for the other family members. The color coding of interface residues highlights the similarities in the binding interfaces of NSP or CAS family members and thus the potential for promiscuous interactions between the 2 families (with the possible exception of the less conserved CASS4). Arrows indicate experimentally confirmed associations between NSP and CAS family members.