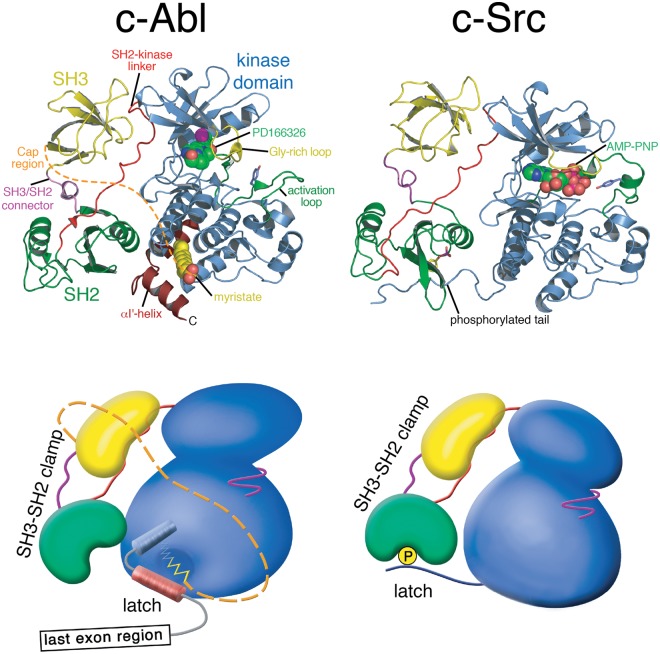
Figure 2.
Structure of autoinhibited Abl and Src. Cartoon representation of autoinhibited Abl in complex with the adenosine triphosphate (ATP)–competitive inhibitor PD166326 (left; PDB entry 1OPK, 18) compared with autoinhibited Src in complex with the ATP analogue AMP-PNP (right; PDB entry 2SRC, 14). Below the cartoon representations of the crystal structures, more schematic representation are used that should illustrate global conformation differences of the 2 kinases. In both kinases, the SH3-SH2 domain unit forms a clamp that inhibits the kinase domain. In Src, the tyrosine-phosphorylated tail binding to the SH2 domain latches the clamp. The myristoyl group of Abl serves as a latch for the SH3-SH2 clamp by inducing a conformational switch in the C-terminal kinase domain helix that gates clamp binding.